Neuropathological Study of the Dorsal Raphe Nuclei in Late-Life Depression and Alzheimer’s Disease With and Without Depression
Abstract
OBJECTIVE: Few studies have examined pathological changes in serotonergic neurons in depression, particularly in elderly patients and in elderly patients in which depression occurs in dementia. The authors hypothesized that greater neurofibrillary pathology and fewer serotonergic neurons would be found in the dorsal raphe nuclei in depressed elderly subjects, compared with nondepressed elderly subjects, and in Alzheimer’s disease patients with depression, compared to Alzheimer’s disease patients without depression. METHOD: In a postmortem study, immunocytochemistry and two-dimensional image analysis were used to measure neuronal density and neuritic pathology in serotonergic neurons in the dorsal raphe nuclei of elderly subjects with primary major depression (N=14), elderly Alzheimer’s disease patients with (N=8) and without (N=7) comorbid depression, and nondepressed elderly comparison subjects (N=10). RESULTS: No differences in neuritic pathology or neuronal density were found between the subjects with primary major depression and the nondepressed comparison subjects. The Alzheimer’s disease subjects showed markedly fewer serotonergic neurons and associated higher levels of neuritic pathology, compared with the subjects with primary depression and the nondepressed comparison subjects, but the Alzheimer’s disease subjects with comorbid major depression did not differ from the Alzheimer’s disease subjects without depression on these measures. CONCLUSIONS: The study found no evidence of a loss of serotonergic neurons or of neuritic pathology in the dorsal raphe nuclei in older people with depression, with or without comorbid Alzheimer’s disease. These findings suggest that if serotonergic dysfunction occurs in older depressed subjects, it is not due to neuronal loss in the brainstem. Pathophysiological changes may lie elsewhere, such as in the frontal-subcortical circuits.
Depression and dementia are the two most common psychiatric disorders in elderly patients, and Alzheimer’s disease is the most common cause of dementia in this group. Depression occurs together with Alzheimer’s disease in about 20% of Alzheimer’s disease patients (1) and is associated with more rapid cognitive decline (2) and a higher mortality rate (3) than are found in Alzheimer’s disease patients without depression. Depression that is comorbid with Alzheimer’s disease shows a poor response to treatment (4).
In addition, depression in older people shows a limited response to current treatment regimes. Even with treatment, good outcome at 1 year is found in only about 60% of elderly subjects with depression (5). The poor prognosis of depression in older people with and without Alzheimer’s disease may be due to the limited knowledge about its etiology and pathogenesis.
It is well established that abnormalities in serotonergic neurotransmission are central to the pathophysiology of depression in younger adults, but whether any underlying pathological changes are associated with these abnormalities is less clear (6). Reports from postmortem studies of serotonin 5-HT1A and 5-HT2A receptors in depression have varied, and these studies have included small numbers of subjects and a high proportion of suicide victims, who may not adequately represent the wider population of persons with major depression (7). Some postmortem studies measuring the serotonin 5-HT transporter in depression have reported deficits in the occipital cortex and hippocampus (8) and in the frontal cortex (9), and a single photon emission computed tomography study using [123I]β-CIT has demonstrated a lower concentration of 5-HT transporter in the brainstem in depressed patients, compared with healthy subjects (10). However, other researchers have reported no such differences (11). A few neuropathological studies of the locus ceruleus in depression have shown neuronal deficits (12, 13), and one report has described a neuronal deficit in the ventral subnuclei of the dorsal raphe nucleus in younger adults with depression (14). However, to our knowledge, no studies of the raphe nuclei in late-life depression have been conducted to determine whether similar effects occur in the brainstem somata of the serotonergic neurons.
Neuropathology studies of the raphe system in Alzheimer’s disease have demonstrated a marked general neuronal deficit and an excess of neuritic pathology (15). Other reports, using staining for tryptophan hydroxylase, have shown a large deficit of serotonergic neurons in both the dorsal and median raphe nuclei (16). Although these studies demonstrated dysfunction in the serotonergic system in Alzheimer’s disease, they did not distinguish Alzheimer’s disease subjects with and without comorbid depression. Neurochemical studies have had more mixed results. One study found marked deficits in 5-HT2 receptors in the frontal, temporal, and cingulate cortices of Alzheimer’s disease patients but only moderate deficits or no differences in 5-HT1 receptors (17). Studies of the 5-HT transporter have shown no differences (17) or a deficit in both the frontal and temporal cortices (18) in patients with Alzheimer’s disease. These varied findings may merely reflect an underlying heterogeneity in the population of Alzheimer’s disease subjects, but they might also represent real differences in the proportion of depressed and nondepressed Alzheimer’s disease subjects in the study samples. Direct comparisons of such subgroups are needed to determine whether serotonergic neurons in depressed Alzheimer’s disease subjects have a higher burden of pathological change.
We conducted a postmortem neuropathological study to examine the dorsal raphe nuclei in late-life major depression and in Alzheimer’s disease with and without comorbid major depression. We hypothesized that there would be an excess of neurofibrillary pathology and fewer serotonergic neurons in the dorsal raphe nuclei in depressed subjects, compared with nondepressed subjects, and in Alzheimer’s disease subjects with comorbid depression, compared to those without comorbid depression.
Method
Clinical Assessment
Tissue samples were obtained from the Newcastle (U.K.) Brain Tissue Bank. All subjects were age 60 or older at the time of death. Full approval for this postmortem study was granted by the Newcastle and North Tyneside local research ethics committee, and consent for postmortem examination was obtained from each subject’s next of kin. Alzheimer’s disease subjects had been prospectively assessed in a longitudinal study of dementia and had met the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association for probable Alzheimer’s disease (19) and had an Mini-Mental State Examination (MMSE) (20) score of >7 at initial assessment. A postmortem diagnosis of definite Alzheimer’s disease was made by using the criteria of the Consortium to Establish a Registry for Alzheimer’s Disease (21). The Alzheimer’s disease subjects had had annual research assessments, including assessment with the cognitive section of the Cambridge Mental Disorders of the Elderly Examination (22), and had been assessed every 6 months with the MMSE and the Cornell Scale for Depression in Dementia (23). Subjects who had scored >10 on the Cornell Scale for Depression in Dementia were assessed by a research psychiatrist to determine whether they met the DSM-III-R criteria for major depression or the DSM-IV criteria for major depressive disorder (ignoring the exclusion criterion of organic causation). Nondepressed Alzheimer’s disease subjects did not meet the DSM criteria for depression at any time point and had Cornell scale scores of <10. Depressed subjects had suffered at least one well-documented episode of DSM-III-R major depression or DSM-IV major depressive disorder, as determined by the treating clinician and by two independent research psychiatrists who reviewed the subjects’ case notes. Subjects were excluded if they had ever met the DSM-IV criteria for dementia, schizophrenia, or another severe mental illness. Subjects with cognitive impairment during depressive episodes were not excluded, provided the impairment was regarded clinically as secondary to the depressed mood and the postmortem examination of the brain showed no neuropathological evidence of changes consistent with dementia. We obtained information from the case notes on the subjects’ history of treatment with antidepressant medication and ECT. The comparison subjects had never had a depressive episode, showed no evidence of a dementing illness, and had been capable of independent living. Postmortem assessment of the comparison subjects also showed no evidence of known causes of dementia.
Tissue Preparation and Identification of Subnuclei
After the postmortem examination, the brainstem was fixed in a 10% buffered formalin solution and embedded in paraffin. The dorsal raphe nucleus was sampled at the level of the trochlear nucleus, and three sections were taken for analysis from each subject. Three series of sections, each consisting of a 6×10-μm section, a 2×20-μm section, and a 3×30-μm section, were cut. From each series, the seventh (20-μm) section was marked with Nissl stain to assist in identification of landmarks, and the adjacent sixth (10-μm) section was used for immunocytochemistry. Slides were coded so that all analyses could be carried out by evaluators who were blind to the subject’s diagnosis.
The dorsal raphe nucleus and its subnuclei were identified by using a standard brainstem atlas (24) and the cytoarchitectural study of the dorsal raphe nucleus by Baker et al. (25). The overall distribution of both Nissl-stained neurons and neurons that were immunoreactive to tryptophan hydroxylase (in tissue from comparison subjects and depressed subjects) corresponded well with these descriptions. The dorsal raphe nucleus was located in the central gray matter ventral to the cerebral aqueduct and fourth ventricle, with most of the nucleus dorsal to the medial longitudinal fasciculi. As previously described (25), the organization and arrangement of the individual subnuclei of the dorsal raphe nucleus at this level formed a fountain shape comprising a medial portion and two lateral wings extending along either side of the midline. Within the medial portion, two subnuclei were clearly visible. The interfascicular subnucleus was located between the medial longitudinal fasciculi, and the ventral subnucleus was immediately dorsal to the interfascicular subnucleus. Demarcation of these two subnuclei was aided by the apparent morphology of the interfascicular subnucleus neurons, which included a preponderance of elongated fusiform neurons that were aligned parallel with the midline. Within the lateral wings of the dorsal raphe nucleus, another two subnuclei were clearly visible: the ventrolateral subnucleus, which was located dorsolateral to the trochlear nucleus, and the dorsal subnucleus, which was immediately dorsal to the ventrolateral subnucleus and just ventral to the floor of the cerebral aqueduct. The ventrolateral subnucleus was easily identified because its densely packed neurons formed two prominent clusters on either side of the midline. The ventral border of the ventrolateral subnucleus was clearly separated from the trochlear nucleus by a narrow cell-free strip. In comparison, the neurons of the dorsal subnucleus were more scattered and occupied the largest volume of these subnuclei. The dorsal subnucleus was bilaterally organized but joined in the midline. Although neurons of the dorsal subnucleus merged with those of the ventrolateral subnucleus, the two subnuclei were clearly demarcated by their differing densities of neurons that were immunoreactive to tryptophan hydroxylase. As in previous studies (25), the dorsal subnucleus exhibited the lowest density of neurons, while the ventrolateral subnucleus had the highest density of neurons. These findings were evident in each of the three diagnostic groups (the comparison, depression, and Alzheimer’s disease groups).
Immunocytochemistry
Serotonergic cells were identified by staining for tryptophan hydroxylase, which is found only in serotonergic neurons (26). The extent of neurofibrillary pathology was quantified by staining for the microtubule-associated tau protein, the key component of neurofibrillary tangles in Alzheimer’s disease (27). Tissue sections were stained by using standard immunocytochemistry. After dewaxing in xylene and rehydration through graded alcohols, the sections were microwaved at 450 W for three 5-minute bursts in a 0.01M citrate buffer solution (pH 6) to optimize antigen retrieval (28). Endogenous peroxidase activity in the tissue was neutralized by washing the sections in a 3% solution of H2O2 in methanol for 15 minutes. After blocking with an appropriate serum, sections were incubated overnight at 4°C in a moist chamber with a monoclonal antibody to tryptophan hydroxylase (mouse IgG3 diluted to a ratio of 1:500) (Sigma-Aldrich Ltd., Dorset, U.K.). After the sections were washed, they were incubated for 30 minutes with an appropriate secondary antibody, then for 30 minutes with avidin-biotinylated horseradish peroxidase complex (serum, secondary antibody, and ABC complex from the “Elite” Vectastain ABC kit, PK-6101) (Vector Laboratories, Peterborough, U.K.). The tissue sections were then placed for 5 minutes in a 10mM phosphate buffered saline solution (pH 7.2) in which the proportion of diaminobenzidine was 0.025% and the proportion of H2O2 was 0.066%. The sections were given three washes in a phosphate-buffered saline solution between all incubation steps except between application of the serum and application of the primary antibody. After the development of the diaminobenzidine, the sections were washed in tap water for 10 minutes, and the procedure was repeated with a second monoclonal antibody to tau (mouse IgG1 diluted to a ratio of 1:4000) (Sigma-Aldrich Ltd.). The microwave retrieval was not repeated. The chromagen used was Vector Immuno Purple (Vector Laboratories), which produced an intense purple precipitate. The rater distinguished the brown-stained cells containing tryptophan hydroxylase easily from the purple-stained cells containing the tau protein, a method more reliable than any automated procedure. After they were washed with tap water, the sections were mounted in synthetic resin.
Analysis of Raphe Pathology
The 10-μm sections were visualized by using a Hitachi light microscope (Hitachi Denshi, Leeds, U.K.), and images were captured by using a JVC charge-coupled device (CCD) color camera (model TK-1070E) (JVC Professional, London). The areas of interest (the subdivisions of the dorsal raphe nucleus) were carefully identified, as described earlier, and demarcated under low power (×4 objective). All cells with positive staining for tryptophan hydroxylase and all cells with positive staining for tau in the demarcated areas were then counted under higher magnification (×25 objective). Thus, all cells with positive staining for tryptophan hydroxylase (the serotonergic cells) and all cells with positive staining for tau (cells with neuritic pathology) in each of the three sections of the dorsal raphe nucleus in each subject were counted. The mean number of serotonergic neurons counted per subject was 318. A mean count per unit area was obtained for both cell types, and the proportion of tau-positive serotonergic cells in each of the three sections was also calculated. Reliability testing of the image analysis was performed by using the data for five randomly chosen subjects, who were assessed on three separate occasions. The procedure had high intrarater reliability (coefficient of variation): 1.6% for evaluation of serotonergic cell density (cells/mm2), 1.6% for evaluation of tau-positive cell density (cells/mm2), and 2.1% for the evaluation of proportion of tau-positive cells.
Statistical Analysis
All statistical analyses were performed by using the SPSS software program, version 9.0 (SPSS, Chicago). The Shapiro-Wilk test of normality and Levene’s test of homogeneity of variances were performed to determine the normality of the data and the equivalence of variances, respectively. Square root transformations were performed if necessary, and comparisons were made by using analysis of variance (ANOVA), which was followed by post hoc unpaired t tests or chi-square tests, as appropriate. The effects of possible confounders were explored by using ANOVA and Pearson’s product-moment correlation.
Results
Fourteen subjects with primary depression, 15 subjects with Alzheimer’s disease, and 10 comparison subjects who met the inclusion criteria were identified. Their characteristics are summarized in Table 1. Twelve of the 14 depressed subjects and eight of the 10 comparison subjects had been included in our previous studies of the frontal subcortical circuits in depression (brain tissue was not available for the remaining subjects in all areas) (29, 30). No significant differences in sex or postmortem interval were found between the three groups, but the groups differed in age and duration of tissue fixation. The depressed subjects were significantly younger than the Alzheimer’s disease subjects, whereas tissue from the Alzheimer’s disease subjects had a significantly shorter mean duration of fixation, compared to tissue from both the comparison subjects and the depressed subjects. Consequently, age and duration of tissue fixation were used as covariates in the analyses.
Findings for the Total Dorsal Raphe Nucleus
The three groups differed in serotonergic cell density, tau-positive cell density, and the proportion of tau-positive cells (Table 1). Post hoc testing revealed that the Alzheimer’s disease subjects had a significantly lower mean serotonergic cell density—about 45% lower—than the comparison subjects and the depressed subjects. In contrast, the Alzheimer’s disease subjects had significantly higher (about ninefold higher) mean tau-positive cell density and a significantly higher proportion of tau-positive cells, relative to the comparison subjects and the depressed subjects. Tau-positive cell density was highly negatively correlated with serotonergic cell density (r=–0.57, df=37, p<0.001). Post hoc testing revealed no significant differences in tau-positive cell density between the comparison group and the depressed group.
We carefully examined possible confounding effects on these findings. Age was correlated with serotonergic cell density (r=–0.39, df=37, p<0.02) and with tau-positive cell density (r=0.38, df=37, p<0.02), and tau-positive cell density was correlated with duration of tissue fixation (r=–0.34, df=37, p<0.04), but both age and duration of fixation were included as covariates in the analyses reported earlier. No correlations were found between postmortem interval and either serotonergic cell density or tau-positive cell density (r<0.14, df=37, p>0.38). Within the depressed group there was no correlation of either serotonergic cell density or tau-positive cell density with age at onset of depression (r<0.05, df=11, p>0.80) or any differences in serotonergic cell density or tau-positive cell density between those with an early onset of depression (before age 60 years, N=5) and those with a late onset (N=9) (F<1.70, df=11, p>0.23). We also examined the effects of a history of treatment with psychotropic medication (tricyclic antidepressants, selective serotonin reuptake inhibitors, antipsychotic drugs, and benzodiazepines) and ECT on the findings, but we found no evidence of any effect of treatment on either tau-positive cell density (F<1.79, df=1, 12, p>0.21) or serotonergic cell density (F<0.26, df=1, 12, p>0.85).
Findings for the Dorsal Raphe Nucleus Subnuclei
Each of the four subnuclei examined showed a similar pattern to that found for the whole dorsal raphe nucleus. These data are presented in Table 2.
Dorsal subnucleus
The three groups differed significantly in mean serotonergic cell density, mean tau-positive cell density, and mean proportion of tau-positive cells in the dorsal subnucleus. Post hoc testing revealed that the Alzheimer’s disease subjects had a significantly lower mean serotonergic cell density, a significantly higher mean tau-positive cell density, and a significantly greater mean proportion of tau-positive cells in the dorsal subnucleus, relative to the comparison subjects and the depressed subjects. Post hoc testing also revealed no significant differences in these measures between the comparison group and the depressed group.
Ventral subnucleus
The three groups differed significantly in mean serotonergic cell density, mean tau-positive cell density, and mean proportion of tau-positive cells in the ventral subnucleus. Post hoc testing revealed that the Alzheimer’s disease subjects had a significantly lower mean serotonergic cell density, a significantly higher mean tau-positive cell density, and a significantly greater mean proportion of tau-positive cells in the ventral subnucleus, relative to the comparison subjects and the depressed subjects. Post hoc testing again revealed no significant differences in these measures between the comparison group and the depressed group.
Ventrolateral subnucleus
The three groups differed significantly in mean serotonergic cell density, mean tau-positive cell density, and mean proportion of tau-positive cells in the ventrolateral subnucleus. Post hoc testing revealed that the Alzheimer’s disease subjects had a significantly lower mean serotonergic cell density, a higher mean tau-positive cell density, and a greater mean proportion of tau-positive cells in the ventrolateral subnucleus, relative to the comparison subjects and the depressed subjects. Post hoc testing revealed no significant differences in these measures between the comparison group and the depressed group.
Interfascicular nucleus
The three groups differed significantly in mean serotonergic cell density, mean tau-positive cell density, and mean proportion of tau-positive cells in the interfascicular nucleus. Post hoc testing revealed that the Alzheimer’s disease subjects had a significantly lower mean serotonergic cell density, a significantly higher mean tau-positive cell density, and a significantly greater mean proportion of tau-positive cells in the interfascicular nucleus, relative to the comparison subjects and the depressed subjects. Post hoc testing revealed no significant differences in these measures between the comparison group and the depressed group.
Findings for the Alzheimer’s Disease Group
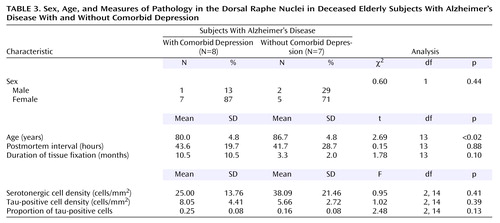
The 15 Alzheimer’s disease subjects consisted of eight subjects who had comorbid depression and seven subjects with no depression (Table 3). Analysis revealed no significant differences between the two groups in sex, postmortem interval, or duration of tissue fixation. However, the groups differed significantly in age; the subjects with Alzheimer’s disease and comorbid depression were significantly younger. Because of this difference, age was used as a covariate in the analyses of data for the Alzheimer’s disease groups.
Compared to the Alzheimer’s disease subjects without depression, the Alzheimer’s disease subjects with comorbid depression had a 34% lower mean serotonergic cell density, a 1.4-fold higher mean tau-positive cell density, and a 1.6-fold higher mean proportion of tau-positive cells in the total dorsal raphe nucleus. The difference for the mean proportion of tau-positive cells just reached significance (t=2.16, df=13, p=0.05). However, in analyses with age as a covariate, this difference was not significant (Table 3). The results for each of the four subnuclei were similar to each other (data not shown) and similar to the results for the total dorsal raphe nucleus.
Discussion
To our knowledge, this study is the first examination of neuropathology in the raphe nuclei in elderly subjects with major depression and the first to investigate pathological changes within the raphe nuclei in Alzheimer’s disease subjects with and without depression. Contrary to our hypothesis, the depressed subjects showed no differences in raphe pathology (neuronal deficits and accumulation of neurofibrillary tangles) relative to the comparison subjects. Similarly, within the Alzheimer’s disease group, there were no differences in raphe pathology between the subjects with depression and those without depression.
The absence of any significant pathology in the dorsal raphe nucleus associated with depression in this study is surprising, given the reported neurochemical abnormalities in the serotonergic system in depression and the recent report of Baumann and colleagues (14), who conducted a morphometric postmortem study of the dorsal raphe nucleus in depression and found a significant 31% deficit in the number of neurons, although they did not specifically examine serotonergic neurons. It may be important that their subjects had a mean age of 50 years, compared with the mean age of 71 years in this study. The contrasting findings provide further evidence that the pathophysiology of depression in later life may be different from that of depression in younger adults. We previously reported higher levels of expression of cell adhesion molecules in the dorsolateral prefrontal cortex in late-life depression (30) and found an associated higher density of glia in the gray matter (31). These findings contrast with postmortem findings of deficits in glia and in neuronal size and density in the dorsolateral prefrontal cortex in younger adults (32). The higher concentration of glia in the older subjects appears to be due to cerebrovascular disease and ischemia, as we found higher concentrations in atheromatous disease (33) and hypoxia-ischemia in the white matter lesions in our subjects (34). In younger adults it has been proposed that the glial changes may be associated with elevated levels of corticosteroids in depressive illness, which could lead to a loss of trophic support for neurons (35). If this hypothesis is correct, the findings of Baumann and colleagues for younger adults suggest that this mechanism might affect the dorsal raphe nucleus as well as the prefrontal cortex. In contrast, in late-life depression, cerebrovascular disease might lead to tissue ischemia and associated gliosis in the prefrontal cortex, especially in the dorsolateral prefrontal cortex, but the brainstem may not be affected. If these differences are real, they may have important implications for treatment.
As expected, the Alzheimer’s disease subjects had significantly greater raphe pathology (neuronal deficit and accumulation of neurofibrillary tangles) than the age-matched comparison subjects or elderly depressed subjects. The finding of a marked deficit of serotonergic neurons in the dorsal raphe nucleus in Alzheimer’s disease is consistent with previous reports, and the magnitude of the deficit (about 45%) is also similar (16, 36). In this study we also showed that neuritic pathology is highly negatively correlated with this deficit of serotonergic cells, suggesting that this Alzheimer’s disease pathology leads to the loss of serotonergic neurons. Halliday and colleagues (37) reported a similar finding in their study of serotonergic neurons in the median raphe nuclei. However, we did not find that the Alzheimer’s disease subjects with depression had a significant additional deficit of these neurons, after we controlled for the subjects’ age. This finding suggests either that the pathophysiology underlying depression in Alzheimer’s disease is distinct from the pathophysiology that causes the cognitive decline leading to the dementia or that the Alzheimer’s disease process precipitates depression as a result of its effects on other brain areas, such as the prefrontal cortex. Further studies are needed to investigate these possibilities, but the evidence for primary depressive illness in older people suggests that cerebrovascular pathology affecting the frontal-subcortical circuitry may be an important factor in depression in people with dementia.
Because this study was based on case notes, we were not able to examine fully all of the possible confounding factors, but it does not appear that our findings are related to basic demographic, histological, or treatment variables. The study benefited from detailed clinical assessments of the subjects, which increased our confidence in the diagnoses, and the relatively large number of subjects for a postmortem investigation. While replication of the findings in older depressed subjects is important, we are confident that our negative findings are robust. Thus, it appears that the pathological causes and anatomical substrates of depression in later life lie outside the dorsal raphe nucleus.
 |
 |
 |
Received Aug. 6, 2003; revision received Oct. 14, 2003; accepted Oct. 31, 2003. From the School of Neurology, Neurobiology and Psychiatry, University of Newcastle upon Tyne, Newcastle upon Tyne; the Institute for Ageing and Health, University of Newcastle upon Tyne; and the Department of Pathology, University of Sheffield, Sheffield, U.K. Address reprint requests to Dr. Thomas, Wolfson Research Centre, Institute for Ageing and Health, Newcastle General Hospital, Newcastle upon Tyne NE4 6BE, UK; [email protected] (e-mail). The authors thank Arthur Oakley and Janet Slade for their help in completing this study.
1. Ballard C, Neill D, O’Brien J, McKeith IG, Ince P, Perry R: Anxiety, depression and psychosis in vascular dementia: prevalence and associations. J Affect Disord 2000; 59:97–106Crossref, Medline, Google Scholar
2. Ritchie K, Touchon J, Ledesert B: Progressive disability in senile dementia is accelerated in the presence of depression. Int J Geriatr Psychiatry 1998; 13:459–461Crossref, Medline, Google Scholar
3. Burns A, Lewis G, Jacoby R, Levy R: Factors affecting survival in Alzheimer’s disease. Psychol Med 1991; 21:363–370Crossref, Medline, Google Scholar
4. O’Brien JT, Ballard C, Winblad B (eds): Controlled Clinical Trials Reports: Alzheimer’s Disease. London, Science Press, 2000Google Scholar
5. Cole MG, Bellavance F: The prognosis of depression in old age. Am J Geriatr Psychiatry 1997; 5:4–14Crossref, Medline, Google Scholar
6. McAllister-Williams RH, Ferrier IN, Young AH: Mood and neuropsychological function in depression: the role of corticosteroids and serotonin. Psychol Med 1998; 28:573–584Crossref, Medline, Google Scholar
7. Meltzer CC, Smith G, DeKosky ST, Pollock BG, Mathis CA, Moore RY, Kupfer DJ, Reynolds CF III: Serotonin in aging, late-life depression, and Alzheimer’s disease: the emerging role of functional imaging. Neuropsychopharmacology 1998; 18:407–430Crossref, Medline, Google Scholar
8. Perry EK, Marshall EF, Blessed G, Tomlinson BE, Perry RH: Decreased imipramine binding in the brains of patients with depressive illness. Br J Psychiatry 1983; 142:188–192Crossref, Medline, Google Scholar
9. Leake A, Fairbairn AF, McKeith IG, Ferrier IN: Studies on the serotonin uptake binding site in major depressive disorder and control post-mortem brain: neurochemical and clinical correlates. Psychiatry Res 1991; 39:155–165Crossref, Medline, Google Scholar
10. Malison RT, Price LH, Berman R, van Dyck CH, Pelton GH, Carpenter L, Sanacora G, Owens MJ, Nemeroff CB, Rajeevan N, Baldwin RM, Seibyl JP, Innis RB, Charney DS: Reduced brain serotonin transporter availability in major depression as measured by [123I]-2 beta-carbomethoxy-3 beta-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry 1998; 44:1090–1098Crossref, Medline, Google Scholar
11. Bligh-Glover W, Kolli TN, Shapiro-Kulnane L, Dilley GE, Friedman L, Balraj E, Rajkowska G, Stockmeier CA: The serotonin transporter in the midbrain of suicide victims with major depression. Biol Psychiatry 2000; 47:1015–1024Crossref, Medline, Google Scholar
12. Chan-Palay V, Asan E: Quantitation of catecholamine neurons in the locus coeruleus in human brains of normal young and older adults and in depression. J Comp Neurol 1989; 287:357–372Crossref, Medline, Google Scholar
13. Arango V, Underwood MD, Mann JJ: Fewer pigmented locus coeruleus neurons in suicide victims: preliminary results. Biol Psychiatry 1996; 39:112–120Crossref, Medline, Google Scholar
14. Baumann B, Bielau H, Krell D, Agelink MW, Diekmann S, Wurthmann C, Trubner K, Bernstein HG, Danos P, Bogerts B: Circumscribed numerical deficit of dorsal raphe neurons in mood disorders. Psychol Med 2002; 32:93–103Crossref, Medline, Google Scholar
15. Yamamoto T, Hirano A: Nucleus raphe dorsalis in Alzheimer’s disease: neurofibrillary tangles and loss of large neurons. Ann Neurol 1985; 17:573–577Crossref, Medline, Google Scholar
16. Chen CP, Eastwood SL, Hope T, McDonald B, Francis PT, Esiri MM: Immunocytochemical study of the dorsal and median raphe nuclei in patients with Alzheimer’s disease prospectively assessed for behavioural changes. Neuropathol Appl Neurobiol 2000; 26:347–355Crossref, Medline, Google Scholar
17. Leake A, Moore PB, Leitch M, Ayre K, Perry RH, Ince PG, Ferrier IN: The serotonergic system in Alzheimer’s disease and normal neocortical post-mortem brain: neurochemical and clinical correlates. Neurol Psychiatry Brain Res 1993; 2:53–59Google Scholar
18. Palmer AM, Wilcock GK, Esiri MM, Francis PT, Bowen DM: Monoaminergic innervation of the frontal and temporal lobes in Alzheimer’s disease. Brain Res 1987; 401:231–238Crossref, Medline, Google Scholar
19. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM: Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34:939–944Crossref, Medline, Google Scholar
20. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
21. Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L: The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), part II: standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 1991; 41:479–486Crossref, Medline, Google Scholar
22. Roth M, Tym E, Mountjoy CQ, Huppert FA, Hendrie H, Verma S, Goddard R: CAMDEX: a standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry 1986; 149:698–709Crossref, Medline, Google Scholar
23. Alexopoulos GS, Abrams RC, Young RC, Shamoian CA: Cornell Scale for Depression in Dementia. Biol Psychiatry 1988; 23:271–284Crossref, Medline, Google Scholar
24. Paxinos G, Huang XF: Atlas of the Human Brainstem. San Diego, Academic Press, 1995Google Scholar
25. Baker KG, Halliday GM, Tork I: Cytoarchitecture of the human dorsal raphe nucleus. J Comp Neurol 1990; 301:147–161Crossref, Medline, Google Scholar
26. Boadle-Biber MC: Regulation of serotonin synthesis. Prog Biophys Mol Biol 1993; 60:1–15Crossref, Medline, Google Scholar
27. Goedert M: Tau protein and the neurofibrillary pathology of Alzheimer’s disease. Trends Neurosci 1993; 16:460–465Crossref, Medline, Google Scholar
28. Cuevas EC, Bateman AC, Wilkins BS, Johnson PA, Williams JH, Lee AH, Jones DB, Wright DH: Microwave antigen retrieval in immunocytochemistry: a study of 80 antibodies. J Clin Pathol 1994; 47:448–452Crossref, Medline, Google Scholar
29. Thomas AJ, Ferrier IN, Kalaria RN, Woodward SA, Ballard C, Oakley A, Perry RH, O’Brien JT: Elevation in late-life depression of intercellular adhesion molecule-1 expression in the dorsolateral prefrontal cortex. Am J Psychiatry 2000; 157:1682–1684Link, Google Scholar
30. Thomas AJ, Ferrier IN, Kalaria RN, Davis S, O’Brien JT: Cell adhesion molecule expression in the dorsolateral prefrontal cortex and anterior cingulate cortex in major depression in the elderly. Br J Psychiatry 2002; 181:129–134Crossref, Medline, Google Scholar
31. Davis S, Thomas A, Perry R, Oakley A, Kalaria RN, O’Brien JT: Glial fibrillary acidic protein in late life major depressive disorder: an immunocytochemical study. J Neurol Neurosurg Psychiatry 2002; 73:556–560Crossref, Medline, Google Scholar
32. Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA: Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry 1999; 45:1085–1098Crossref, Medline, Google Scholar
33. Thomas AJ, Ferrier IN, Kalaria RN, Perry RH, Brown A, O’Brien JT: A neuropathological study of vascular factors in late-life depression. J Neurol Neurosurg Psychiatry 2001; 70:83–87Crossref, Medline, Google Scholar
34. Thomas AJ, O’Brien JT, Davis S, Ballard C, Barber R, Kalaria RN, Perry RH: Ischemic basis for deep white matter hyperintensities in major depression: a neuropathological study. Arch Gen Psychiatry 2002; 59:785–792Crossref, Medline, Google Scholar
35. Cotter DR, Pariante CM, Everall IP: Glial cell abnormalities in major psychiatric disorders: the evidence and implications. Brain Res Bull 2001; 55:585–595Crossref, Medline, Google Scholar
36. Zweig RM, Ross CA, Hedreen JC, Steele C, Cardillo JE, Whitehouse PJ, Folstein MF, Price DL: The neuropathology of aminergic nuclei in Alzheimer’s disease. Ann Neurol 1988; 24:233–242Crossref, Medline, Google Scholar
37. Halliday GM, McCann HL, Pamphlett R, Brooks WS, Creasey H, McCusker E, Cotton RG, Broe GA, Harper CG: Brain stem serotonin-synthesizing neurons in Alzheimer’s disease: a clinicopathological correlation. Acta Neuropathol 1992; 84:638–650Crossref, Medline, Google Scholar



