Balance and Gait Deficits in Schizophrenia Compounded by the Comorbidity of Alcoholism
Abstract
OBJECTIVE: Alcoholism carries a liability of balance and gait instability that persists with sobriety. Such deficits are less well documented in schizophrenia and may be compounded by comorbidity with alcoholism, which is prevalent in schizophrenia. METHOD: The authors administered quantitative ataxia tests to 10 patients comorbid for schizophrenia and alcohol dependence/abuse, 10 nonalcoholic patients with schizophrenia, 24 nonschizophrenic patients with alcohol dependence, and 27 age-matched comparison men. RESULTS: All three patient groups were impaired relative to the comparison subjects. The comorbid group was significantly more impaired than the alcoholic group on most tests and was more impaired than the schizophrenia patients, especially when tested with eyes open. CONCLUSIONS: Rigorous quantitative testing revealed gait and balance deficits in schizophrenia, even without alcohol dependence, and exacerbated deficits in schizophrenia comorbid with alcoholism. The enhancement of postural stability expected with visual information was dampened in comorbid patients, implicating compromised sensorimotor integrative abilities.
Disequilibrium and gait peculiarities were featured in classic descriptions (1, 2) of patients with schizophrenia, even before the advent of neuroleptics, and are detectable with clinical neurological assessment (3–5). Ataxia of stance and gait is also salient behavioral sequelae to chronic alcohol abuse (4, 6, 7) and may arise from compromise of the anterior superior cerebellar vermis (postmortem [6, 8, 9] and in vivo [10]).
Comorbidity of ataxia with alcohol use disorders is highly prevalent in schizophrenia (e.g., references 11, 12, 13) and has an adverse impact on schizophrenia’s clinical course (for reviews, see references 14, 15). Such comorbidity exacerbates existing deficits of brain volume in prefrontal and anterior temporal gray matter (16) and produces deficits in the anterior superior cerebellar vermis (17) and pons (18), even in schizophrenia patients with remote histories of alcoholism and low levels of lifetime alcohol consumption relative to alcoholic patients without schizophrenia. Given this heightened vulnerability for patients comorbid for both schizophrenia and alcoholism, especially in the anterior superior cerebellar vermis, we assessed gait and balance by using quantitative ataxia tests to determine whether comorbid patients would show greater postural instability than patients with either condition alone.
Method
All subjects were men and gave written informed consent to participate in the research. The patients were recruited from a Veterans Administration medical center and included 10 with a DSM-III-R axis I diagnosis of schizophrenia only, 10 comorbid for DSM-III-R-defined schizophrenia and alcohol dependence or abuse, and 24 with DSM-III-R-defined alcohol dependence only. No patient met criteria for any other axis I disorder, with the exception of two of the comorbid patients who had a history of past cannabis abuse.
The patients with schizophrenia were tested for gait and balance while inpatients; the patients with alcoholism were tested when returning for follow-up studies (19, 20). Alcoholic patients reported a wide range of days of sobriety (1 to 1,994, median=204) at the time of testing. The comorbid patients also reported a wide range of days of sobriety (17 to 3,285, median=88). The length of sobriety did not differ between these groups (Mann-Whitney U=117, df=32, p=0.91).
All schizophrenia patients had been treated pharmacologically; when tested, five schizophrenia and seven comorbid patients were taking atypical antipsychotic medications, three schizophrenic and two comorbid patients were taking typical antipsychotic medications, one schizophrenic patient was temporarily unmedicated, and the medication status was unknown for one schizophrenic and one comorbid patient. Current symptom severity was evaluated in patients with schizophrenia by using the Brief Psychiatric Rating Scale (BPRS) (21), administered by two raters with established reliability. Lifetime alcohol consumption was assessed by using a semistructured interview (22–25) in all patients with alcoholism and healthy comparison subjects, nine of the 10 comorbid patients, and nine of the 10 patients with schizophrenia only.
The healthy comparison group was recruited from the local community and comprised 27 men selected from a larger group of 61 men (e.g., reference 10) to age-match the patient groups. All had been screened to exclude any axis I disorder, substance abuse in the year before the study, and alcohol consumption of more than four drinks a day for over a month.
One-way analyses of variance (ANOVAs) of demographic variables (Table 1) across all groups yielded significant differences in education (F=8.39, df=3, 67, p<0.0001), general intelligence (estimated with the National Adult Reading Test [26]) (F=3.76, df=3, 67, p<0.05), and total lifetime consumption of alcohol (F=33.98, df=3, 65, p<0.0001) but not age (F=1.00, df=3, 67, p=0.42) or handedness (F=1.80, df=3, 65, p=0.15), measured quantitatively (27). Follow-up Scheffé tests (alpha=0.05) revealed that the group with alcoholism consumed more alcohol than all other groups. Differences between the schizophrenia and healthy comparison groups were not significant; the comorbid patients tended to drink more than the healthy comparison subjects (t=1.88, df=34, p<0.10) and the patients with schizophrenia (t=1.89, df=16, p<0.10). The three patient groups had equivalent years of education and National Adult Reading Test IQs. The comorbid group did not differ significantly from the schizophrenia group in BPRS scores.
Gait and static balance were assessed with the Walk-a-Line Ataxia Battery (28), consisting of three parts, each performed first with eyes open and then with eyes closed. First, the subject stood with feet placed heel to toe and arms folded across the chest for 60-second trials (“stand heel to toe”). Next, the subject stood on one foot for 30-second trials (“stand on one foot”). Finally, the subject walked heel to toe for 10 steps (“walk heel to toe”). Each condition was performed twice unless the subject achieved a perfect score on the initial trial.
Because gait and balance performance can decline with age, we applied linear regression to data from the larger group of healthy comparison subjects spanning the adult age range (20 to 70 years) to derive age-corrected standardized z scores for each participant (10). Values for each group reflect the extent to which it deviated from age norms. Group differences in performance for each composite were assessed with four-group, one- or two-way ANOVAs and follow-up Scheffé tests. Associations between variables were assessed with Pearson’s correlations and confirmed with Spearman’s tests because of small group sizes.
Results
Composite scores for the eyes-open and eyes-closed conditions (Figure 1) showed significant effects of group (F=15.97, df=3, 65, p=0.0001) and condition (F=13.24, df=1, 65, p=0.0005); the interaction (F=6.41, df=3, 65, p=0.0007) indicated greater deficits in all three patient groups with eyes open than with eyes closed. With one exception (alcoholics with eyes closed), follow-up tests showed that all patient groups had deficits in both conditions relative to the performance of the healthy comparison group, and the comorbid group had deficits relative to the alcoholic group.
Composite scores for the three test parts—stand heel to toe, stand on one foot, and walk heel to toe (Figure 2)—irrespective of visual condition, also showed effects of group (F=14.89, df=3, 132, p=0.0001) and test part (F=3.70, df=2, 132, p=0.028) but no interaction effect (F=1.70, df=2, 132, p=0.13). Scheffé tests indicated significantly lower scores than the comparison group on all three measures by the comorbid group, the two static balance measures by the schizophrenia group, and one measure (stand on one foot) by the alcoholic group. The comorbid group had significantly greater deficits than the alcoholic group for standing heel to toe and standing on one foot. Overall, the extent of the comorbid group’s deficit was the sum of the schizophrenia group’s and alcoholism group’s deficits.
Ataxia scores were not associated with lifetime alcohol consumption or length of sobriety in alcoholics or comorbid patients. Further, BPRS scores did not predict ataxia scores in either schizophrenia group, with or without alcoholism comorbidity. An ANOVA for factors of medication type (typical versus atypical) and diagnosis (schizophrenia versus comorbid) revealed no effect of medication or a medication-by-diagnosis interaction.
Discussion
This study identified deficits in balance and gait relative to age norms in patients with alcoholism, even following substantial periods of sobriety, as well as in patients with schizophrenia but without a history of alcoholism. The patients with schizophrenia who had also met lifetime criteria for alcoholism showed a compounded deficit (effect size across all tests ranged from SD=1.1 to SD=3.6), hence, greater than that observed in either condition alone (effect sizes ranging from SD=0.6 to SD=1.2 for alcoholism and SD=0.9 to SD=2.1 for schizophrenia). Comorbid patients were significantly more impaired than alcoholics, even though they had consumed only one-eighth of the alcohol over their lifetime relative to the nonpsychotic alcoholics. The group included only men; whether a similar pattern occurs in women remains to be studied.
The lack of statistically significant performance differences between comorbid patients and schizophrenia patients is consistent with a recent report also employing balance tests requiring standing in the Romberg position and walking heel to toe (29). In that study, although the two schizophrenia groups did not show statistically significant differences in performance, the mean score of the comorbid group (where higher scores were in the impaired direction) was more than double that of the group with schizophrenia but without alcoholism.
Gait performance in comorbid and schizophrenia patients could have been affected by antipsychotic medication. Some studies have reported that patients taking neuroleptics perform significantly less well than untreated patients on fine motor coordination (e.g., reference 30). Others have reported that atypical neuroleptics are associated with better motor coordination than typical neuroleptics in patients with schizophrenia (31). In this group, schizophrenia and comorbid patient groups did not differ in medication type, and there were no significant medication type effects or group-by-medication interactions. Thus, the relatively poorer performance of the comorbid patients than the schizophrenia patients is probably not solely a medication effect.
The additive untoward effect of alcoholism on an already compromised schizophrenic motor system (4, 32) parallels our earlier reports of exacerbated regional brain volume in the prefrontal cortex (16), cerebellar vermis, hemisphere gray matter (17), and pons (18) in patients comorbid for both conditions. A possible mechanism underlying this excessive postural instability is disruption of the cerebellar systems required for maintaining posture and balance, also present in schizophrenia-alcoholism comorbidity (17). The results of the present study provide support for the working hypothesis that alcohol abuse, which occurs in about half the population of schizophrenia patients (33), results in increased vulnerability of the brains of patients with schizophrenia to the exogenous toxin—alcohol. A manifestation of this increased vulnerability, readily demonstrated by standardized gait assessment, places comorbid patients at risk of falling. Contributing to this liability is a possible compromise of sensorimotor integration, implicated by the especially reduced ability of the comorbid group to stabilize balance with visual cues.
 |
Presented in part at the 41st annual meeting of the College of Neuropsychopharmacology, Dec. 8–12, 2002, San Juan, Puerto Rico. Received March 8, 2003; revision received Aug. 19, 2003; accepted Aug. 25, 2003. From the Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine; and the Neuroscience Program, SRI International, Menlo Park, Calif. Address reprint requests to Dr. Sullivan, Department of Psychiatry and Behavioral Sciences, 401 Quarry Rd., Stanford University School of Medicine, Stanford, CA 94305–5723; [email protected] (e-mail). Supported by grants from the National Institute on Alcohol Abuse and Alcoholism (AA-10723 and AA-05965) and NIMH (MH-58007 and MH-30854).
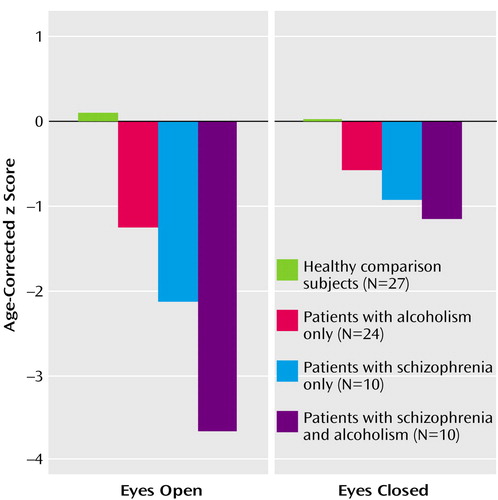
Figure 1. Composite Ataxia Performance for Healthy Comparison Subjects, Patients With Alcoholism Only, Patients With Schizophrenia Only, and Patients With Both Disordersa
aANOVAs showed significant group (F=15.97, df=3, 65, p=0.0001) and condition (F=13.24, df=1, 65, p=0.0005) effects as well as a significant interaction (F=6.41, df=3, 65, p=0.0007). The eyes-open condition provided a more sensitive measure of disease-related deficits than the eyes-closed condition because the comparison group performed near the rating ceiling with eyes open but not with eyes closed.
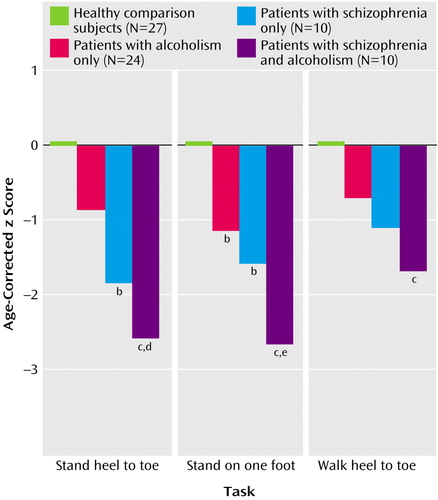
Figure 2. Performance on Three Ataxia Tests, Collapsed Over Eyes-Open and Eyes-Closed Conditions, for Healthy Comparison Subjects, Patients With Alcoholism Only, Patients With Schizophrenia Only, and Patients With Both Disordersa
aANOVAs showed significant group (F=14.89, df=3, 132, p=0.0001) and test type (F=3.70, df=2, 132, p<0.03) effects.
bSignificantly different from comparison subjects (p<0.005, post hoc Scheffé test).
cSignificantly different from comparison subjects (p<0.001, post hoc Scheffé test).
dSignificantly different from alcoholism-only group (p<0.05, post hoc Scheffé test).
eSignificantly different from alcoholism-only group (p<0.01, post hoc Scheffé test).
1. Kraepelin E: Dementia Praecox and Paraphrenia (1919). Translated by Barclay RM; edited by Robertson GM. Huntington, NY, Robert E Krieger, 1971Google Scholar
2. Bleuler E: Dementia Praecox or the Group of Schizophrenias (1908). Translated by Zinkin J. New York, International Universities Press, 1950Google Scholar
3. Manschreck TC, Ames D: Neurologic features and psychopathology in schizophrenic disorders. Biol Psychiatry 1984; 19:703–719Medline, Google Scholar
4. Deshmukh A, Rosenbloom MJ, Pfefferbaum A, Sullivan EV: Clinical signs of cerebellar dysfunction in schizophrenia and alcoholism. Schizophr Res 2002; 57:281–291Crossref, Medline, Google Scholar
5. Sanders RD, Keshavan MS, Forman SD, Pieri JN, McLaughlin N, Allen DN, van Kammen DP, Goldstein G: Factor structure of neurologic examination abnormalities in unmedicated schizophrenia. Psychiatry Res 2000; 95:237–243Crossref, Medline, Google Scholar
6. Victor M, Adams RD, Collins GH: The Wernicke-Korsakoff Syndrome and Related Neurologic Disorders Due to Alcoholism and Malnutrition, 2nd ed. Philadelphia, FA Davis, 1989Google Scholar
7. Sullivan EV, Rosenbloom MJ, Pfefferbaum A: Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res 2000; 24:611–621Crossref, Medline, Google Scholar
8. Victor M, Adam RD, Mancell EL: A restricted form of cerebellar degeneration occurring in alcoholic patients. Arch Neurol 1959; 1:577–688Crossref, Google Scholar
9. Torvik A, Torp S: The prevalence of alcoholic cerebellar atrophy: a morphometric and histological study of an autopsy material. J Neurol Sci 1986; 75:43–51Crossref, Medline, Google Scholar
10. Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A: Cerebellar volume decline in normal aging, alcoholism, and Korsakoff’s syndrome: relation to ataxia. Neuropsychology 2000; 14:341–352Crossref, Medline, Google Scholar
11. Cuffel BJ: Prevalence estimates of substance abuse in schizophrenia and their correlates. J Nerv Ment Dis 1992; 180:589–592Crossref, Medline, Google Scholar
12. Fowler IL, Carr VJ, Carter NT, Lewin TJ: Patterns of current and lifetime substance use in schizophrenia. Schizophr Bull 1998; 24:443–455Crossref, Medline, Google Scholar
13. Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK: Comorbidity of mental disorders with alcohol and other drug abuse: results from the Epidemiologic Catchment Area (ECA) study. JAMA 1990; 264:2511–2518Crossref, Medline, Google Scholar
14. Buckley PF: Substance abuse in schizophrenia: a review. J Clin Psychiatry 1998; 59(suppl 3):26–30Google Scholar
15. Dixon L: Dual diagnosis of substance abuse in schizophrenia: prevalence and impact on outcomes. Schizophr Res 1999; 35(suppl):S93-S100Google Scholar
16. Mathalon DH, Pfefferbaum A, Lim KO, Rosenbloom MJ, Sullivan EV: Compounded brain volume deficits in schizophrenia-alcoholism comorbidity. Arch Gen Psychiatry 2003; 60:245–252Crossref, Medline, Google Scholar
17. Sullivan EV, Deshmukh A, Desmond JE, Mathalon DH, Rosenbloom MJ, Lim KO, Pfefferbaum A: Contribution of alcohol abuse to cerebellar volume deficits in men with schizophrenia. Arch Gen Psychiatry 2000; 57:894–902Crossref, Medline, Google Scholar
18. Sullivan EV, Rosenbloom MJ, Serventi KL, Deshmukh A, Pfefferbaum A: Effects of alcohol dependence comorbidity and antipsychotic medication on volumes of the thalamus and pons in schizophrenia. Am J Psychiatry 2003; 160:1110–1116Link, Google Scholar
19. Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO: Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res 1995; 19:1177–1191Crossref, Medline, Google Scholar
20. Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO: A controlled study of cortical gray matter and ventricular changes in alcoholic men over a five year interval. Arch Gen Psychiatry 1998; 55:905–912Crossref, Medline, Google Scholar
21. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Crossref, Google Scholar
22. Skinner HA: Development and Validation of a Lifetime Alcohol Consumption Assessment Procedure. Toronto, Addiction Research Foundation, 1982Google Scholar
23. Skinner HA, Sheu WJ: Reliability of alcohol use indices: the lifetime drinking history and the MAST. J Stud Alcohol 1982; 43:1157–1170Crossref, Medline, Google Scholar
24. Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Lane B, Ha CN, Rosenbloom MJ, Sullivan EV: Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res 1992; 16:1078–1089Crossref, Medline, Google Scholar
25. Pfefferbaum A, Rosenbloom MJ, Crusan K, Jernigan TL: Brain CT changes in alcoholics: the effects of age and alcohol consumption. Alcohol Clin Exp Res 1988; 12:81–87Crossref, Medline, Google Scholar
26. Nelson HE: The National Adult Reading Test (NART). Windsor, UK, Nelson, 1982Google Scholar
27. Crovitz HF, Zener KA: Group test for assessing hand and eye dominance. Am J Psychol 1962; 75:271–276Crossref, Medline, Google Scholar
28. Fregly AR, Graybiel A, Smith MS: Walk on floor eyes closed (WOFEC): a new addition to an ataxia test battery. Aerosp Med 1972; 43:395–399Medline, Google Scholar
29. Allen D, Goldstein G, Forman S, Keshavan M, van Kammen D, Sanders R: Neurologic examination abnormalities in schizophrenia with and without a history of alcoholism. Neuropsychiatry Neuropsychol Behav Neurol 2000; 13:184–187Medline, Google Scholar
30. Cleghorn JM, Kaplan RD, Szechtman B, Szechtman H, Brown GM: Neuroleptic drug effects on cognitive function in schizophrenia. Schizophr Res 1990; 3:211–219Crossref, Medline, Google Scholar
31. Gallhofer B, Bauer U, Lis S, Krieger S, Gruppe H: Cognitive dysfunction in schizophrenia: comparison of treatment with atypical antipsychotic agents and conventional neuroleptic drugs. Eur Neuropsychopharmacol 1996; 6(suppl):S13-S20Google Scholar
32. Sullivan EV, Fama R, Shear PK, Cahn-Weiner D, Stein M, Zipursky RB, Pfefferbaum A: Motor sequencing deficits in schizophrenia: a comparison with Parkinson’s disease. Neuropsychology 2001; 15:342–350Crossref, Medline, Google Scholar
33. Regier DA, Narrow WE, Rae DS, Manderscheid RW, Locke BZ, Goodwin FK: The de facto US mental and addictive disorders service system: Epidemiologic Catchment Area prospective 1-year prevalence rates of disorders and services. Arch Gen Psychiatry 1993; 50:85–94Crossref, Medline, Google Scholar



