Apathy in Schizophrenia: Reduced Frontal Lobe Volume and Neuropsychological Deficits
Abstract
OBJECTIVE: Apathy is a common negative symptom in schizophrenia. The authors investigated neuropsychological performance and regional brain volumes in schizophrenia patients with high versus low levels of apathy. METHOD: Schizophrenia patients with low apathy levels (N=18) and high apathy levels (N=20) and 12 healthy comparison subjects completed neuropsychological testing as well as magnetic resonance imaging scanning to obtain lobar volumes after total intracranial volume was controlled. RESULTS: The high apathy group scored lower than comparison subjects on rapid visuomotor sequencing and verbal learning/recall. The high apathy group had lower performance IQ scores than the low apathy and comparison groups. Only the high apathy group showed significantly reduced bilateral frontal lobe volumes relative to comparison subjects; both schizophrenia patient groups showed bilateral temporal lobe volume reductions. CONCLUSIONS: The present findings are consistent with studies in other disorders showing frontal lobe involvement in apathy.
Apathy is common in patients with frontal-subcortical or right hemisphere damage due to various etiologies (1, 2). Neuroimaging studies of neurological patients have reported that apathy is associated with a frontal-subcortical circuitry abnormality (3, 4). Apathy has also been associated with executive dysfunction that is not accounted for by depressed mood, which is again consistent with a frontal-subcortical abnormality (5, 6).
Apathy is a common negative symptom in schizophrenia that has been associated with poor compliance or poor ability to benefit from treatment (7, 8). The neurobiological underpinnings of apathy in schizophrenia have not been systematically investigated, although frontal lobe abnormalities have been shown in both structural and functional imaging studies of deficit syndrome schizophrenia (9, 10). In the present study, we compared schizophrenia patients with low versus high levels of apathy and healthy subjects on neuropsychological tests sensitive to executive and right hemisphere dysfunction; lobar volumes from structural magnetic resonance imaging (MRI) scans were also compared. It was predicted that schizophrenia patients with high apathy levels would perform more poorly on measures of executive function and right hemisphere functioning and would have reduced frontal lobe volume.
Method
Subjects included 18 patients with schizophrenia or schizoaffective disorder exhibiting no or mild apathy (low apathy group), 20 exhibiting moderate to severe apathy (high apathy group), and 12 healthy comparison subjects. Patients were excluded if they had a comorbid axis I diagnosis, history of neurological illness, head injury with loss of consciousness, or systemic illness with potential cognitive sequelae. Apathy grouping was based on score on the apathy subscale of the Scale for the Assessment of Negative Symptoms (SANS) (11). The low apathy group had scores of 0 or 1, while the high apathy group had scores of 2 or higher. All but two patients were receiving stable doses of medication; four patients were receiving divalproex rather than an antipsychotic. Clinical assessment with the Structured Clinical Interview for DSM-IV, Brief Psychiatric Rating Scale (BPRS), Schedule for Assessment of Positive Symptoms (SAPS), and the SANS was completed with two authors present (L.A.F., T.W.M.); consensus ratings were used. Written informed consent was obtained from all subjects.
Subjects completed neuropsychological examination and structural MRI that used a 1.5-Tesla GE magnet (parameters: spoiled grass sequence, TE=13 msec, TR=38 msec, flip angle=45°, number of excitations=1), which yielded a series of 124 contiguous 1.5-mm coronal slices. Total intracranial and right and left hemisphere volumes for the frontal, temporal and parietal lobes (in cubic centimeters) were calculated by using BRAINS, a standardized semiautomated software package developed at the University of Iowa; detailed methods, including boundary definitions, have been previously reported (12).
Group differences were analyzed by using analysis of variance (ANOVA), multiple analysis of variance (MANOVA) using Wilks’s lambda, and nonparametric statistics as appropriate. Significant ANOVAs were followed by post hoc analyses that used Tukey’s least significant difference test. Lobar volumes were adjusted for total intracranial volume by using a regression model, including all participants in the estimation of the relationship between regional volumes and intracranial volume. Two-tailed comparisons with significance level set at p<0.05 were used.
Results
There were no differences between schizophrenia patients with high versus low levels of apathy in terms of age (mean=35.0 [SD=13.3] and 33.2 years [SD=8.1], respectively), WRAT-III reading score (mean=97.9 [SD=13.3] and 92.8 [SD=14.4]), handedness (right-handed: N=17 for both; left-handed: N=3 and 1), sex (14 men, six women and 15 men, three women), or chlorpromazine equivalents (mean=496.3 [SD=351.7] and 421.6 mg [SD=293.5]). They also had similar BPRS total scores (mean=44.5 [SD=11.2] and 47.2 [SD=6.6]) and BPRS depression scores (mean=1.9 [SD=1.3] and 1.8 [SD=1.3]). The high apathy group had higher scores than did the low apathy group on the SAPS (mean=10.5 [SD=3.3] versus 7.0 [SD=4.6]; t=2.69, df=36, p=0.01) and SANS (mean=12.3 [SD=3.0] versus 5.8 [SD=3.3]; t=6.38, df=36, p=0.001). Healthy subjects were comparable in age (mean=31.7, SD=8.7), WRAT reading score (mean=102.4, SD=12.5), handedness (all were right-handed) and sex (11 men, one woman).
A MANOVA that included all neuropsychological test scores was significant (F=1.80, df=24, 72, p=0.03). Further analyses revealed that the high apathy group scored lower than comparison subjects on Trail Making Test Part A (F=4.10, df=2, 47, p=0.02) and Part B (F=3.97, df=2, 47, p=0.03) as well as the California Verbal Learning Test measures of learning (F=5.95, df=2, 47, p=0.005) and delayed recall (F=3.59, df=2, 47, p=0.04). The high apathy group also had a lower performance IQ score than the low apathy and comparison groups (F=3.26, df=2, 47, p=0.04).
The high but not low apathy group showed significantly reduced adjusted volumes relative to the healthy comparison subjects in the right frontal lobe (high apathy group: mean=195.7 [SD=19.6]; comparison subjects: mean=215.8 [SD=17.8]) (F=5.53, df=2, 47, p=0.007) and left frontal lobe (high apathy group: mean=184.8 [SD=19.9]; comparison subjects: mean=200.4 [SD=16.2]) (F=3.48, df=2, 47, p=0.04) (Figure 1). The high apathy and low apathy schizophrenia patient groups both had significantly smaller adjusted volumes than did the comparison subjects in the right temporal lobe (mean=107.5 [SD=6.0] and 111.7 [SD=6.2] versus 118.5 [SD=6.5], respectively; F=11.71, df=2, 47, p=0.001) and left temporal lobe (mean=107.2 [SD=5.6] and 109.2 [SD=8.3] versus 118.8 [SD=8.0]; F=10.20, df=2, 47, p=0.001). A similar but nonsignificant difference was noted for adjusted left and right parietal lobe volumes. SAPS score did not correlate with any of the dependent measures in the combined patient group.
Discussion
In this study, schizophrenia patients with high levels of apathy had poorer visuomotor sequencing and verbal learning and memory, lower performance IQ, and bilateral frontal lobe volume reductions. In contrast, both patient groups performed more poorly than comparison subjects on psychomotor speed and naming, had lower verbal and full-scale IQ scores, and showed bilateral temporal lobe volume reduction, consistent with other studies of schizophrenia (13). Lack of effort during testing is unlikely to account for these findings, since a generalized cognitive deficit was not found in the high relative to low apathy group. Findings were unrelated to level of depression or overall severity of psychopathology. However, we cannot rule out the possibility that differences in overall negative and positive symptom severity may have contributed to the present findings. With this caveat, findings are generally consistent with studies of neurological disorders showing frontal-subcortical or right hemisphere involvement in apathy (2).
Impaired sequencing ability in our high apathy group is consistent with prefrontal-thalamus-basal ganglia circuitry involvement in sequencing (14), the finding of reduced frontal lobe volume in this group, and neuroimaging evidence of disruption of this circuitry in patients with schizophrenia with prominent negative symptoms (10, 15). The lower performance IQ in the high apathy patients is consistent with studies showing a relationship between right hemisphere integrity and negative symptoms in schizophrenia (15, 16). The specific cognitive processes through which frontal-subcortical or right hemisphere dysfunction may lead to apathy in schizophrenia remain to be elucidated. Studies of neurological patients have implicated impaired allocation of attention to novel stimuli (17, 18) or disruption of the ability to create internal referents that permit selection of appropriate responses to incoming stimuli (19) as the basis for apathy. Studies of these cognitive processes in relation to apathy in schizophrenia would likely be informative.
In conclusion, results of this preliminary study suggest that apathy in schizophrenia is associated with a frontal-subcortical or right hemisphere abnormality. Further studies with larger sample sizes and more comprehensive neuropsychological test batteries would be helpful in determining the generalizability of these findings. Finally, further evaluation of the structural integrity of frontal lobe subregions may be informative, given that the cingulate gyrus has been particularly associated with apathy in other populations.
Received Jan. 7, 2003; revision received July 28, 2003; accepted July 31, 2003. From the Neuropsychology Program and Brain Imaging Laboratory, Department of Psychiatry, Dartmouth Medical School and New Hampshire Hospital. Address reprint requests to Dr. Roth, Brain Imaging Laboratory, Department of Psychiatry, Dartmouth Medical School, One Medical Center Dr., Lebanon, NH 03756-0001; [email protected] (e-mail). Supported by grants from the National Alliance for Research on Schizophrenia and Depression, the Ira DeCamp Foundation, and the New Hampshire Hospital.
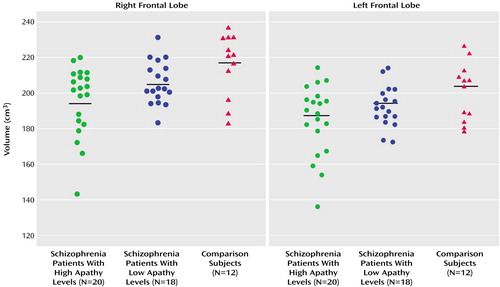
Figure 1. Adjusted Right and Left Frontal Lobe Volumes of Schizophrenia Patients With Low Versus High Levels of Apathy and Healthy Comparison Subjectsa
aApathy level classified by score on the SANS apathy subscale (low: score of 0 or 1; high: score ≥2).
1. Aarsland D, Larsen JP, Lim NG, Janvin C, Karlsen K, Tandberg E, Cummings JL: Range of neuropsychiatric disturbances in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 1999; 67:492–496Crossref, Medline, Google Scholar
2. McAllister TW: Apathy. Semin Clin Neuropsychiatry 2000; 5:275–282Crossref, Medline, Google Scholar
3. Okada K, Kobayashi S, Yamagata S, Takahashi K, Yamaguchi S: Poststroke apathy and regional cerebral blood flow. Stroke 1997; 28:2437–2441Crossref, Medline, Google Scholar
4. Migneco O, Benoit M, Koulibaly PM, Dygai I, Bertogliati C, Desvignes P, Robert PH, Malandain G, Bussiere F, Darcourt J: Perfusion brain SPECT and statistical parametric mapping analysis indicate that apathy is a cingulate syndrome: a study in Alzheimer’s disease and nondemented patients. Neuroimage 2001; 13:896–902Crossref, Medline, Google Scholar
5. Isella V, Melzi P, Grimaldi M, Iurlaro S, Piolti R, Ferrarese C, Frattola L, Appollonio I: Clinical, neuropsychological, and morphometric correlates of apathy in Parkinson’s disease. Mov Disord 2002; 17:366–371Crossref, Medline, Google Scholar
6. McPherson S, Fairbanks L, Tiken S, Cummings JL, Back-Madruga C: Apathy and executive function in Alzheimer’s disease. J Int Neuropsychol Soc 2002; 8:373–381Crossref, Medline, Google Scholar
7. Tattan TM, Creed FH: Negative symptoms of schizophrenia and compliance with medication. Schizophr Bull 2001; 27:149–155Crossref, Medline, Google Scholar
8. Torres A, Mendez LP, Merino H, Moran EA: Improving social functioning in schizophrenia by playing the train game. Psychiatr Serv 2002; 53:799–801Link, Google Scholar
9. Buchanan RW, Breier A, Kirkpatrick B, Elkashef A, Munson RC, Gellad F, Carpenter WT Jr: Structural abnormalities in deficit and nondeficit schizophrenia. Am J Psychiatry 1993; 150:59–65Link, Google Scholar
10. Lahti AC, Holcomb HH, Medoff DR, Weiler MA, Tamminga CA, Carpenter WT Jr: Abnormal patterns of regional cerebral blood flow in schizophrenia with primary negative symptoms during an effortful auditory recognition task. Am J Psychiatry 2001; 158:1797–1808Link, Google Scholar
11. Andreasen NC: Negative symptoms in schizophrenia: definition and reliability. Arch Gen Psychiatry 1982; 39:784–788Crossref, Medline, Google Scholar
12. Andreasen NC, Rajarethinam R, Cizadlo T, Arndt S, Swayze VW II, Flashman LA, O’Leary DS, Ehrhardt JC, Yuh WTC: Automatic atlas-based volume estimation of human brain regions from MR images. J Comp Assist Tomogr 1996; 20:98–106Crossref, Medline, Google Scholar
13. Shenton ME, Dickey CC, Frumin M, McCarley RW: A review of MRI findings in schizophrenia. Schizophr Res 2001; 49:1–52Crossref, Medline, Google Scholar
14. Nakahara H, Doya K, Hikosaka O: Parallel cortico-basal ganglia mechanisms for acquisition and execution of visuomotor sequences—a computational approach. J Cogn Neurosci 2001; 13:626–647Crossref, Medline, Google Scholar
15. Potkin SG, Alva G, Fleming K, Anand R, Keator D, Carreon D, Doo M, Jin Y, Wu JC, Fallon JH: A PET study of the pathophysiology of negative symptoms in schizophrenia. Am J Psychiatry 2002; 159:227–237Link, Google Scholar
16. Wible CG, Anderson J, Shenton ME, Kricun A, Hirayasu Y, Tanaka S, Levitt JJ, O’Donnell BF, Kikinis R, Jolesz FA, McCarley RW: Prefrontal cortex, negative symptoms, and schizophrenia: an MRI study. Psychiatry Res 2001; 108:65–78Crossref, Medline, Google Scholar
17. Daffner KR, Mesulam MM, Scinto LF, Acar D, Calvo V, Faust R, Chabrerie A, Kennedy B, Holcomb P: The central role of the prefrontal cortex in directing attention to novel events. Brain 2000; 123(part 5):927–939Google Scholar
18. Daffner KR, Rentz DM, Scinto LF, Faust R, Budson AE, Holcomb PJ: Pathophysiology underlying diminished attention to novel events in patients with early AD. Neurology 2001; 56:1377–1383Crossref, Medline, Google Scholar
19. Godefroy O, Rousseaux M: Novel decision making in patients with prefrontal or posterior brain damage. Neurology 1997; 49:695–701Crossref, Medline, Google Scholar



