Association of Dopamine Transporter Loss in the Orbitofrontal and Dorsolateral Prefrontal Cortices With Methamphetamine-Related Psychiatric Symptoms
Abstract
OBJECTIVE: The authors examined dopamine transporter density in the orbitofrontal cortex, dorsolateral prefrontal cortex, and amygdala in methamphetamine users and assessed the relationship of these measures to the subjects’ clinical characteristics. METHOD: Positron emission tomography with [11C]WIN 35,428 was used to examine the regions of interest in 11 methamphetamine users and nine healthy comparison subjects. Psychiatric symptoms were evaluated with the Brief Psychiatric Rating Scale. RESULTS: Dopamine transporter density in the three regions studied was significantly lower in the methamphetamine users than in the comparison subjects. The lower dopamine transporter density in the orbitofrontal and dorsolateral prefrontal cortex was significantly correlated with the duration of methamphetamine use and the severity of psychiatric symptoms. CONCLUSIONS: Chronic methamphetamine use may cause dopamine transporter reduction in the orbitofrontal cortex, dorsolateral prefrontal cortex, and amygdala in the brain. Psychiatric symptoms in methamphetamine users may be attributable to the decrease in dopamine transporter density in the orbitofrontal cortex and the dorsolateral prefrontal cortex.
Human in vivo imaging studies have documented significant dopamine transporter loss in methamphetamine users (1, 2). In our previous positron emission tomography (PET) study (3), we found significant dopamine transporter reduction in the striatum and nucleus accumbens of methamphetamine users; in addition, this reduction was associated with the duration of methamphetamine use and the severity of positive symptoms. However, our previous study did not address the importance of the orbitofrontal cortex and dorsolateral prefrontal cortex in methamphetamine-associated psychiatric states, although two studies dealing with this issue have been published since the publication of our article (4, 5). A PET study by Volkow et al. (4) assessed the availability of dopamine D2 receptors in methamphetamine users in addition to investigating regional brain glucose metabolism as a marker of brain function. Methamphetamine users had a significantly lower level of D2 receptors in the striatum than was observed in comparison subjects; this decrease was associated with the dysregulation of orbitofrontal cortex function. A functional magnetic resonance imaging (fMRI) study by Paulus et al. (5) demonstrated that methamphetamine users failed to exhibit normal activation in both the orbitofrontal cortex and the dorsolateral prefrontal cortex during a decision-making task. Therefore, we reevaluated dopamine transporter density in the orbitofrontal cortex and dorsolateral prefrontal cortex in methamphetamine users in terms of the extent to which this density was associated with clinical characteristics, including psychotic symptoms, duration of methamphetamine use, and craving for methamphetamine. In the current study, we also considered dopamine transporter density in the amygdala, which has dopaminergic connections with both the orbitofrontal cortex and the dorsolateral prefrontal cortex (6).
Method
This study was approved by the Ethics Committee of the Hamamatsu University School of Medicine. After complete description of the study, written informed consent was obtained from all subjects before study entry.
Eleven male abstinent methamphetamine users (mean age=27.4 years, SD=5.6) and nine healthy male comparison subjects (mean age=26.9 years, SD=4.5) were evaluated in this study. These participants were the same as those in our previous study (3). The comparison subjects had no history of psychiatric disorder or substance abuse. None of the methamphetamine users had a history of psychiatric symptoms before the use of methamphetamine, and none had used any drugs other than methamphetamine. The demographic characteristics of the methamphetamine users have been described elsewhere in detail (3). Briefly, the duration of methamphetamine use ranged from 1 month to 15 years (mean=4.8 years, SD=4.8), and methamphetamine abstinence ranged from 7 days to 1.5 years (mean=5.6 months, SD=5.7). At the time of the PET scan, three methamphetamine users had no psychiatric symptoms, four had persistent anxiety disorder-like symptoms, and four had persistent psychotic symptoms as determined by the Brief Psychiatric Rating Scale (BPRS). The Subjective Craving Scale for Alcohol Dependence was slightly modified and used for the assessment of methamphetamine craving.
To evaluate dopamine transporter density in the orbitofrontal cortex, dorsolateral prefrontal cortex, and amygdala, we reanalyzed the PET scan data from our original study (3). PET scans were performed by using an SHR2400 PET scanner (Hamamatsu Photonics K.K., Hamamatsu, Japan) following intravenous injection of [11C]WIN 35,428. A sequence of 38 PET scans was obtained over 90 minutes. Arterial blood samples were drawn at intervals of 10 seconds to 15 minutes after the tracer injection. On the basis of a three-compartment and four-parameter (K1, k2, k3, k4) model, dopamine transporter density, represented by the binding potential (k3/k4), was calculated as previously described (3, 7). The frontal regions studied here, i.e., the orbitofrontal and dorsolateral prefrontal cortices, are known to be sensitive to the partial volume effect of cortical atrophy due to substance use (8). To minimize the contribution of the partial volume effect, magnetic resonance images were superimposed onto PET images according to previously described procedures (7). Subsequently, by referring to areas on the magnetic resonance images as anatomical landmarks, regions of interest were carefully drawn to avoid the involvement of the sulci.
In order to compare the values of the binding data between methamphetamine users and healthy comparison subjects, the Mann-Whitney U test was used. Correlations between dopamine transporter binding and the clinical parameters (duration of methamphetamine use, duration of abstinence, BPRS score, and craving score) were evaluated with Kendall’s tau. Statistical significance was set at p<0.05.
Results
Dopamine transporter density in the three regions tested was significantly lower in the methamphetamine users than in the comparison subjects (orbitofrontal cortex: mean=0.16 [SD=0.09] versus 0.26 [SD=0.02], respectively [Mann-Whitney U=16.0, N=20, p<0.01]; dorsolateral prefrontal cortex: mean=0.17 [SD=0.07] versus 0.18 [SD=0.03] [Mann-Whitney U=7.0, N=20, p<0.01]; amygdala: mean=0.11 [SD=0.07] versus 0.19 [SD=0.04] [Mann-Whitney U=19.0, N=20, p<0.05]). There was a significant negative correlation between dopamine transporter binding potential in both the orbitofrontal and dorsolateral prefrontal cortices and the duration of methamphetamine use (Figure 1). Similarly, the dopamine transporter binding potential in the two cortical regions showed a significantly negative correlation with the score on the BPRS subscale for positive symptoms. Dopamine transporter binding potential was not significantly correlated with the duration of abstinence or the magnitude of methamphetamine craving in either the orbitofrontal or dorsolateral prefrontal cortex. For the amygdala, the binding potential was not correlated with any of the clinical variables.
Discussion
To our knowledge, this is the first study to report a significant reduction in dopamine transporter density in the orbitofrontal cortex, dorsolateral prefrontal cortex, and amygdala in methamphetamine users. When taken together with the results of our previous study showing a significant decrease in dopamine transporter density for the striatum, nucleus accumbens, and anterior prefrontal cortex (3), it is suggested that chronic intake of methamphetamine leads to dopamine transporter reduction in discrete brain areas that receive dopaminergic input. The dopamine transporter reduction in the orbitofrontal cortex and the dorsolateral prefrontal cortex was significantly associated with the duration of methamphetamine use and with the severity of persistent psychiatric symptoms. This finding further supports our contention that a longer period of methamphetamine use may cause a relatively greater reduction in dopamine transporter density in the brain, a result that may also be closely associated with the severity of psychiatric symptoms. In this study, no correlation was found between dopamine transporter density and the duration of abstinence (mean=6 months). However, this result does not necessarily indicate that the reduction in dopamine transporters is irreversible, since Volkow et al. (9) have provided evidence of a significant recovery of dopamine transporter density with protracted abstinence (mean=17 months).
A recent PET study suggested dopamine D2 receptor-mediated dysregulation in the orbitofrontal cortex of methamphetamine users (4). Moreover, dysfunction of the orbitofrontal cortex and the dorsolateral prefrontal cortex during decision making was demonstrated by a recent fMRI study (5). It is possible that the dopamine transporter may be a specific site damaged by methamphetamine, and the dopaminergic system is likely to be actively involved in the function of the orbitofrontal cortex and dorsolateral prefrontal cortex. However, it remains unknown whether or not the observed dopamine transporter reduction participates in the functional disruption of the orbitofrontal cortex and dorsolateral prefrontal cortex. If there were indeed such a direct influence, determination of its mechanism would be of great interest. Hence, further work in this area is still needed.
Unlike in the orbitofrontal cortex and dorsolateral prefrontal cortex, dopamine transporter density in the amygdala was not correlated with any of the clinical markers discussed here. A larger study would be of benefit, as would the use of a tracer with a higher specific-to-nonspecific binding ratio, in order to clarify the differences between dopamine transporter density in the amygdala and that in the other areas of the brain studied here.
Received Sept. 26, 2002; revision received Jan. 22, 2003; accepted Feb. 6, 2003. From the Department of Psychiatry and Neurology, Hamamatsu University School of Medicine; the Positron Medical Center, Hamamatsu Medical Center, Hamakita, Japan; and the Central Research Laboratory, Hamamatsu Photonics K.K., Hamakita, Japan. Address reprint requests to Dr. Minabe, Department of Psychiatry and Neurology, Hamamatsu University School of Medicine, 1-20-1 Handayama, Hamamatsu, 431-3192 Shizuoka, Japan; [email protected] (e-mail). Supported by a Grant-in-Aid for the Encouragement of Young Scientists from the Ministry of Education of Japan and grants from the Ministry of Health and Welfare of Japan, the Ministry of Science and Technology of Japan, and the Stanley Foundation. The authors thank Charles R. Ashby, Jr., for his suggestions and Shuji Nobezawa and Toshihiko Kanno for technical support.
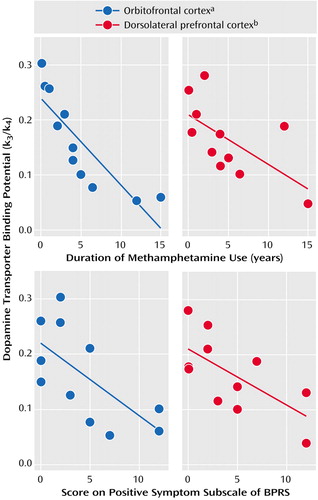
Figure 1. Relationship Between Clinical Variables and Dopamine Transporter Binding Potential in the Orbitofrontal Cortex and Dorsolateral Prefrontal Cortex of Methamphetamine Users (N=11)
aSignificant negative correlation between binding potential and both duration of methamphetamine use (Kendall’s tau=–0.92, df=10, p<0.001) and BPRS positive symptom subscale score (Kendall’s tau=–0.52, df=10, p<0.05).
bSignificant negative correlation between binding potential and both duration of methamphetamine use (Kendall’s tau=–0.55, df=10, p<0.05) and BPRS positive symptom subscale score (Kendall’s tau=–0.48, df=10, p<0.05).
1. McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA: Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci 1998; 18:8417–8422Crossref, Medline, Google Scholar
2. Volkow ND, Chang L, Wang G-J, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding Y-S, Logan J, Wong C, Miller EN: Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry 2001; 158:377–382Link, Google Scholar
3. Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Takei N, Mori N: Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry 2001; 158:1206–1214Link, Google Scholar
4. Volkow ND, Chang L, Wang G-J, Fowler JS, Ding Y-S, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N: Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry 2001; 158:2015–2021Link, Google Scholar
5. Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, Schuckit MA: Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology 2002; 26:53–63Crossref, Medline, Google Scholar
6. Holland PC, Gallagher M: Amygdala circuitry in attentional and representational processes. Trends Cogn Sci 1999; 3:65–73Crossref, Medline, Google Scholar
7. Ouchi Y, Yoshikawa E, Okada H, Futatsubashi M, Sekine Y, Iyo M, Sakamoto M: Alterations in binding site density of dopamine transporter in the striatum, orbitofrontal cortex, and amygdala in early Parkinson’s disease: compartment analysis for β-CFT binding with positron emission tomography. Ann Neurol 1999; 45:601–610Crossref, Medline, Google Scholar
8. Wang GJ, Volkow ND, Roque CT, Cestaro VL, Hitzemann RJ, Cantos EL, Levy AV, Dhawan AP: Functional importance of ventricular enlargement and cortical atrophy in healthy subjects and alcoholics as assessed with PET, MR imaging, and neuropsychologic testing. Radiology 1993; 186:59–65Crossref, Medline, Google Scholar
9. Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J: Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci 2001; 21:9414–9418Crossref, Medline, Google Scholar



