Increase in Prefrontal Cortex Serotonin2A Receptors Following Estrogen Treatment in Postmenopausal Women
Abstract
OBJECTIVE: This study investigated the effect of estrogen on brain serotonin 2A (5-HT2A) receptors in postmenopausal women and whether there was any correlation of receptor changes with cognition and mood. METHOD: Ten postmenopausal subjects underwent positron emission tomography measurements of 5-HT2A receptor binding with [18F]deuteroaltanserin before and after estrogen replacement therapy. RESULTS: 5-HT2A receptor binding was significantly increased after estrogen replacement therapy in the right prefrontal cortex (right precentral gyrus [Brodmann’s area 9], inferior frontal gyrus [Brodmann’s area 47], medial frontal gyrus [Brodmann’s area 6, 10] and the anterior cingulate cortex [Brodmann’s area 32]). In the inferior frontal gyrus [Brodmann’s area 44]), receptor up-regulation was correlated with change in plasma estradiol. Verbal fluency and Trail Making Test performance, but not mood, were significantly improved by estrogen without correlation with receptor changes. CONCLUSIONS: Estrogen increases 5-HT2A receptor binding in human prefrontal regions.
Despite conflicting studies, a recent meta-analysis indicated that estrogen may improve specific cognitive domains, e.g., verbal memory and selected executive cognitions (vigilance, reasoning, motor speed, verbal function) (1). As well as its potential to elevate mood, the estrogen effects may be partly achieved via the serotonin system. Prefrontal serotonin 2A (5-HT2A) receptors, shown to be increased in rodents following estrogen administration (2), are one component relating to cognition/mood and actions of antipsychotics/antidepressants (3). The receptor modulation by estrogen may account for hormonal regulation of cognition/mood or pathophysiology of menstrually related psychiatric disorders. A recent human positron emission tomography (PET) study (4), however, failed to demonstrate a statistically significant increase in brain 5-HT2A receptors after estrogen replacement therapy (17β-estradiol), possibly because of study group size (N=5) and use of a region of interest analysis, which can miss localized change. The current PET study was conducted to verify—by using a voxel-by-voxel image analysis—the hypothesis that estrogen increases human brain 5-HT2A receptors and that the change mediates the cognitive/mood effects of estrogen.
Method
The ten right-handed subjects (mean age=54.5 years, SD=6.6, range=44–68) were postmenopausal women (plasma levels of follicle-stimulating hormone ≥30 IU/liter and no menstrual cycle for at least a year [mean=6.3 years, SD=7.3]) with no history of psychiatric illness according to the Structured Clinical Interview for DSM-III-R, Non-Patient Edition, given as part of the screening evaluation. Subjects were free of estrogen for at least 2 months and psychoactive drugs for a month. Written informed consent was obtained from all subjects. Subjects received estrogen replacement therapy with a transdermal patch (17β-estradiol, 0.075–0.15 mg; mean dose=0.084 mg [SD=0.012]; mean dose at second PET scan=0.093 mg [SD=0.024]) for a mean of 10.2 weeks (SD=1.8). Plasma estradiol levels were measured by radioimmunoassay. Subjects completed a PET scan following assessments before and after estrogen replacement therapy.
Cognitive assessments, typically performed the day before scans, included tests of verbal memory (paragraph recall and verbal paired associates tests from the Wechsler Memory Scale—Revised [5]), executive cognition (Trail Making Test A to assess attention/motor speed and Test B to assess sequencing [6]), and verbal fluency (letters/categories [7]). Mood was assessed on the PET scan days with the depression/dejection subscale of the Profile of Mood States (POMS) (8) and Beck Depression Inventory (9).
Subjects received a bolus injection of [18F]deuteroaltanserin (mean=211.4 MBq, SD=10.1), followed by a 5-hour continuous infusion (bolus-to-infusion rate ratios: mean=3.21, SD=0.01) before a 45-minute PET scan (Posicam 6.5; 21 slices with 5.125-mm interslice distance) (10). Magnetic resonance imaging (MRI) scans were acquired with a GE 1.5-T Signa scanner. PET voxel value of 5-HT2A receptor binding was V3′ (ml/ml)=(specific binding in cortices minus cerebellum activity)/total plasma parent concentration. At equilibrium, V3′ is proportional to Bmax/Kd (test/retest reproducibility: intrasubject change of 14.1%; reliability: intraclass coefficients of variation of 0.86) (10) and is not affected by blood flow. Attenuation-corrected PET images were normalized to Talairach space by using MRI (coregistered to PET by the Automated Imaging Registration [Medx4.0]) and “T1.img” within statistical parametric mapping (SPM 99), followed by smoothing (full width at half maximum=20 mm). This statistical parametric mapping method enabled the evaluation of voxel-by-voxel change in receptor binding between two scans and is more suitable to detect localized change than region of interest analysis.
The SPM 99 statistical t map threshold was 2.82 (p=0.01) for comparison and 1.86 (p=0.05) for correlation with estradiol. All p values were two-tailed, and statistical significance was set at p<0.05 (with/without Bonferroni correction) in SPSS 11.0.
Results
Estrogen replacement therapy significantly increased plasma estradiol levels from baseline to endpoint in all subjects (mean=14.7 pg/ml [SD=9.0] versus 176.5 pg/ml [SD=150.0], respectively; p=0.006). There were no significant differences between the two scans in radiotracer plasma parameters (data not shown). 5-HT2A receptor binding was increased after estrogen replacement therapy in the regions shown in Figure 1. The significant increase of 36.5% (V3′ mean=0.51 ml/ml [SD=0.21] versus mean=0.70 ml/ml [SD=0.26]) (p=0.01) was primarily localized to the right frontal cortex (size=5568 pixels) (corrected p=0.001). The peak t scores were found in the right precentral gyrus (Brodmann’s area 9 [Talairach coordinates: x=40, y=16, z=36]; t=3.79), inferior frontal gyrus (Brodmann’s area 47 [x=50, y=22, z=–2 and x=48, y=22, z=–6]; t=3.74 and 3.71, respectively), medial frontal gyrus (Brodmann’s area 10 [x=4, y=52, z=–6]; t=3.31 and Brodmann’s area 6 [x=16, y=10, z=50]; t=3.31), and anterior cingulate cortex (Brodmann’s area 32 [x=10, y=38, z=16]; t=3.06). Positive and significant correlation with plasma estradiol was found in the right inferior frontal cortex (size 103 pixels) within SPM 99 (peak t=2.63 [x=56, y=6, z=14]; corrected p=0.022).
Significant performance improvement following estrogen replacement therapy was seen for category verbal fluency (baseline: mean=14.8 [SD=3.2], endpoint: mean=18.5 [SD=3.1]; corrected p=0.016) and Trail Making Test A (baseline: mean=34.7 seconds [SD=9.7], endpoint: mean=28.2 seconds [SD=8.3]; corrected p=0.028). No significant improvement was seen in immediate paragraph recall (baseline: mean=19.2 [SD=4.8], endpoint: 17.0 [SD=4.1]), delayed paragraph recall (% retention) (baseline: mean=67.7 [SD=17.9], endpoint: 84.8 [SD=11.4]), or verbal paired associates performance (immediate: mean=12.1 [SD=5.6] versus 15.5 [SD=6.4]; delayed [% retention]: mean=82.5 [SD=22.2] versus 86.3 [SD=17.1]). Baseline mood scales showed euthymic condition (POMS: mean=1.1 [SD=2.1]; Beck Depression Inventory: mean=2.2 [SD=2.7]) and were nonsignificantly decreased after estrogen replacement therapy (p>0.54). These changes in scales were not associated with receptor changes in the above region (p>0.12 in SPSS).
Discussion
The current study found significant, relatively unilateral (right > left) and localized changes in 5-HT2A receptors after estrogen replacement therapy (dorsal to ventral prefrontal regions including dorsolateral and cingulate cortices). Prefrontal 5-HT2A receptors have an action on the pyramidal cells via glutamate release and may well modulate cognitive functions as the stimulation of primate prefrontal. 5-HT2A receptors (caudal area 46 and rostral area 8a) improved spatial working memory (3). The similarity of our finding to the regions where estrogen evoked human brain activity during a working memory task (Brodmann’s area 46, 47) (11) strengthens the possibility that 5-HT2A receptors may be involved in estrogen-induced modulation of these cognitive functions. Subjects’ improvement of executive/prefrontal-related functions (i.e., verbal fluency and Trail Making Test A) may support the possibility. The results are consistent with the meta-analysis (1) that revealed estrogen effects on motor speed and verbal fluency but not on complex attention tests such as Trails B. Unexpected lack of change in verbal memory might stem from the study group size and noncontrolled design. Future studies would need a control condition and direct measurement of working memory. Other limitations include baseline euthymia, which might decrease the likelihood of correlations with receptor change because of floor effects. Last, use of a ligand-specific template for image normalization may have proven to be more reliable for a study of this type.
Presented in part at the 31st annual meeting of the Society for Neuroscience, San Diego, Nov. 10–15, 2001. Received July 15, 2002; revision received Dec. 8, 2002; accepted March 11, 2003. From the Department of Psychiatry, the Department of Pharmacology, and the Department of Nuclear Medicine, Yale University, New Haven, Conn. Address reprint requests to Dr. Kugaya, VA Connecticut Healthcare System–116A2, 950 Campbell Ave., West Haven, CT 06516; [email protected] (e-mail). Supported in part by NIMH grant MH-58620 and by the Japan Foundation for Aging and Health (S. Yamawaki, Hiroshima University, principal investigator). The authors thank the Yale PET Center staff for technical assistance in collecting the data; S. Giddings for subject recruitment; and S. Wasylink, K.A. Czarkowski, and S. Osbourne of the Yale Behavioral Gynecology Clinic for subject assessment and follow-up.
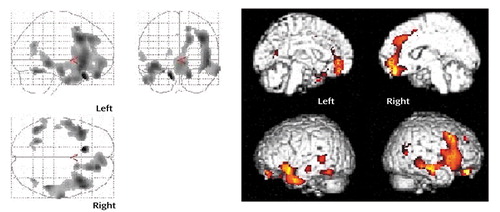
Figure 1. Serotonin 5-HT2A Receptor Binding After Estrogen Administration in Postmenopausal Women (N=10)a
aThe statistical parametric maps show areas where 5-HT2A receptor binding significantly increased after estrogen replacement therapy (threshold t=2.82, p=0.01). Results projected onto three orthogonal two-dimensional planes are shown on the left; darker area indicates higher statistical significance. The images on the right are results for the lateral and medial quadrants of the left and right hemispheres projected onto lateral and medial views of a brain.
1. LeBlanc ES, Janowsky J, Chan BK, Nelson HD: Hormone replacement therapy and cognition: systematic review and meta-analysis. JAMA 2001; 285:1489–1499Crossref, Medline, Google Scholar
2. Sumner BE, Grant KE, Rosie R, Hegele-Hartung C, Fritzemeier KH, Fink G: Effects of tamoxifen on serotonin transporter and 5-hydroxytryptamine(2A) receptor binding sites and mRNA levels in the brain of ovariectomized rats with or without acute estradiol replacement. Brain Res Mol Brain Res 1999; 73:119–128Crossref, Medline, Google Scholar
3. Williams GV, Rao SG, Goldman-Rakic PS: The physiological role of 5-HT2A receptors in working memory. J Neurosci 2002; 22:2843–2854Crossref, Medline, Google Scholar
4. Moses EL, Drevets WC, Smith G, Mathis CA, Kalro BN, Butters MA, Leondires MP, Greer PJ, Lopresti B, Loucks TL, Berga SL: Effects of estradiol and progesterone administration on human serotonin 2A receptor binding: a PET study. Biol Psychiatry 2000; 48:854–860Crossref, Medline, Google Scholar
5. Wechsler D: Wechsler Memory Scale—Revised Manual. New York, Harcourt Brace Jovanovich, 1987Google Scholar
6. Reitan R, Davison L: Validity of the Trailmaking Test as an indication of organic brain damage. Percept Mot Skills 1974; 8:271–276Crossref, Google Scholar
7. Benton A, Hamsher K: Multilingual Aphasia Examination. Iowa City, University of Iowa, 1978Google Scholar
8. McNair DM, Lorr M, Droppleman LF: Manual for the Profile of Mood States. San Diego, Calif, Educational and Industrial Testing Service, 1971Google Scholar
9. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571Crossref, Medline, Google Scholar
10. Soares JC, van Dyck CH, Tan P, Zoghbi SS, Garg P, Soufer R, Baldwin RM, Fujita M, Staley JK, Fu X, Amici L, Seibyl J, Innis RB: Reproducibility of in vivo brain measures of 5-HT2A receptors with PET and [18F]deuteroaltanserin. Psychiatry Res 2001; 106:81–93Crossref, Medline, Google Scholar
11. Berman K, Schmidt P, Rubinow D, Danaceau M, Horn JV, Esposito G, Ostrem J, Weinberger D: Modulation of cognition-specific cortical activity by gonadal steroids: a positron-emission tomography study in women. Proc Natl Acad Sci USA 1997; 94:8836–8841Crossref, Medline, Google Scholar



