Transfer of Olanzapine Into Breast Milk, Calculation of Infant Drug Dose, and Effect on Breast-Fed Infants
Abstract
OBJECTIVE: This study characterized infant drug doses and breast-milk-to-plasma area-under-the-curve ratios for olanzapine and determined plasma concentrations and effects of this drug on breast-feeding infants. METHOD: Seven mother-infant nursing pairs were studied. Olanzapine was measured in plasma and milk with high-performance liquid chromatography over a dose interval (for six patients) or at a single time after dose ingestion (for one patient) at steady state. Infant drug exposure was estimated as the product of an assumed milk production rate and average drug concentration in milk, normalized to body weight, and expressed as a percentage of maternal drug dose, normalized to body weight. RESULTS: The median infant dose of olanzapine ingested through milk was 1.02% of the maternal dose; the median milk-to-plasma area-under-the-curve ratio was 0.38 for the six patients with data collected over the dose interval. Corresponding values in the patient with single-point data were 1.13% and 0.75. Olanzapine was not detected in the plasma of the six infants with an evaluable plasma sample. All of the infants were healthy and experienced no side effects. CONCLUSIONS: Breast-fed infants were exposed to a calculated olanzapine dose of approximately 1%—well below the 10% notional level of concern. In infant plasma, olanzapine was below the detection limit; there were no adverse effects on the infants. These data support the use of olanzapine during breast-feeding. However, the authors recommend that breast-fed infants be monitored closely and the decision to breast-feed be made after individual risk-benefit analysis.
Olanzapine is an atypical antipsychotic agent that acts as an antagonist mainly at dopamine D2, serotonin 2A, and acetylcholine receptors in the CNS (1, 2). It is well absorbed orally but has some first-pass metabolism, which gives it an oral availability of 60.0%–80.0% (3). Peak plasma drug concentrations occur approximately 5 to 8 hours after an oral dose (4, 5). Olanzapine is metabolized extensively in the liver through oxidation (by means of cytochrome P450 [CYP] enzymes CYP 1A2 and CYP 2D6 and flavin mono-oxygenase) and subsequent conjugation (glucuronidation) (6). At least 10 metabolites have been identified, but they either are pharmacologically inactive or have only weak activity in vitro (2, 7). Plasma protein binding is >90.0% and is independent of concentration (8). The β-phase volume of distribution is 16.4 liter/kg, and oral clearance ranges from 12 to 47 liter/hour (7, 8). Elimination half-life ranges from 21 to 54 hours and varies with age, gender, smoking status, and co-medications (7–10).
The incidence of psychiatric disorders is at its peak during the postpartum period, with up to 20% of mothers affected (11). In particular, the rate of postpartum psychosis (largely bipolar mood disorder) is high (12), with most cases occurring within the first month after delivery (13). As a newer atypical antipsychotic agent, with effects on both positive and negative symptoms of schizophrenia (5) and an improved side effect profile (14), olanzapine offers advantages over older antipsychotics. Another important feature is that it has less propensity to raise prolactin concentrations, which indicates that premenopausal women are likely to be more fertile when taking olanzapine than typical antipsychotics (14). Olanzapine may also contribute to mood stabilization in patients with bipolar disorder (15) and is therefore of particular value in postpartum manic and mixed mood states.
The use of olanzapine in the treatment of postpartum psychosis has been limited by sparse data on its transfer into breast milk (16) and on the effects of exposure to the drug on a suckling infant (17). Two other reports of olanzapine use in human lactation (17, 18) have been published. None of the three infants had adverse effects that were attributed to olanzapine exposure. Pharmacokinetic analyses for determination of olanzapine concentrations in maternal blood and milk were not reported. However, olanzapine was not detected (limit of detection=2 μg/liter) in two blood samples taken from one of the suckling infants (18).
This article describes the extent of infant exposure to olanzapine in seven breast-feeding women and briefly documents their infants’ health and development.
Method
Seven breast-feeding women and their infants were enrolled in the study. The study design was approved by the research and ethics committee of King Edward Memorial and Princess Margaret Hospitals, Western Australia, and by the Canterbury ethics committee, Christchurch, New Zealand. Written informed consent was obtained from all participants.
Venous blood samples (5–10 ml, heparinized) were collected from six of the women either with a forearm catheter or by venipuncture five to seven different times across a dose interval of 12 or 24 hours. At approximately the same time intervals, milk samples were obtained with an electric breast pump, and aliquots (10–15 ml) were retained for drug assay. Milk was collected by one of two methods that attempted to provide a mean estimate of olanzapine concentrations in milk over an expression/feeding period. Subjects A–C emptied their breasts of milk, the milk was mixed, the volume was recorded, and a sample from each expression was retained for analysis. Subjects D–F provided milk samples pre- and postfeeding; equal volumes were mixed together in the clinic, and this sample was retained for analysis. For three subjects, the entire study was conducted in the hospital, while plasma and milk sampling after 8 hours occurred in the homes of the remaining three. For the seventh woman, a single set of pre- and postfeeding milk samples was collected, together with a plasma sample, 18 hours after her morning drug dose. Six of the women gave consent for a venous blood sample (0.5–2.0 ml, heparinized) to be taken from their infants. Infant health and well-being were evaluated by inquiry of all the mothers, together with a clinical examination by a specialist neonatologist or general practitioner (patients B, C, D, E, F, and G) using developmental screening and assessment instruments (19), the Denver Development Screening Test (20), or a Griffiths developmental assessment (21) in five cases. Infant body weight for age was checked against gender-specific population percentile graphs (22).
Olanzapine standard material was donated by Eli Lilly Australia, Pty. Ltd., West Ryde, Australia, and mexiletine (internal standard material) by Boehringer Ingelheim, Pty. Ltd., North Ryde, Australia. All solvents and chemicals were of analytical or high-performance liquid chromatography grade.
High-performance liquid chromatography was used to quantify olanzapine in plasma (23) and milk (16). The intra- and interday coefficients of variation for the plasma assay were 4.6% and 2.4%, and 8.2% and 3.9% at 6 μg/liter and 120 μg/liter, respectively. For milk, the intra- and interday coefficients of variation were 17.3%, 6.3%, and 3.4%, and 19.2%, 6.3%, and 3.3% at 1 μg/liter, 6 μg/liter, and 120 μg/liter, respectively. The limit of quantitation was approximately 1 μg/liter for both plasma and milk. However, for infant plasma, the limit of quantitation was sometimes as high as 5 μg/liter because less than a 1-ml sample was available. Data were summarized as means (ranges) or medians (25th and 75th percentile ranges), as appropriate.
The area under the concentration time curves for a dose interval (12 or 24 hours) was calculated with Topfit 2.0 (log trapezoidal rule) (24) for plasma and rectangular areas for milk (Σ concentration by collection time) (25). Milk-to-plasma ratios were calculated from data for area under the curve for six patients and single milk and plasma concentration measurements for one patient. An infant milk intake of 0.15 liter/kg/day was assumed (26); this value was multiplied by the average milk concentration (area under the curve0–τ/dose interval, in which τ=dose interval; patients A–F) or a single-point milk concentration (patient G) to give the absolute infant dose of olanzapine in μg/kg/day. The infant dose was then expressed as a percentage of the weight-normalized maternal dose (μg/kg/day).
Results
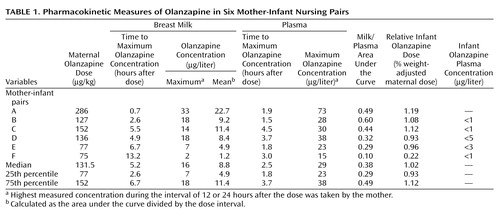
The mean age of the women was 30 years (range=23–39), and their mean body weight was 71 kg (range=59–110). Their infants consisted of three boys and four girls with a mean age of 2.4 months (range=0.1–4.3) and a mean weight of 5.3 kg (range=4.2–6.3). The median dose of olanzapine ingested by the women was 7.5 mg/day (range=5–20) or 127 μg/kg/day (range=75–286). Pharmacotherapy with olanzapine had begun a mean of 114 days (range=19–395) before the study day, and all participants were considered to be at steady state at the time of study. Patients A and F took olanzapine during both pregnancy and lactation, while all of the other patients began taking the drug in the postpartum period because of the development of acute psychosis.
The plasma and milk concentration time profile for subject D is shown in Figure 1, and the pharmacokinetic descriptors for subjects A–F appear in Table 1. The median maximum olanzapine plasma concentration of 29 μg/liter occurred at a median time of 2.5 hours after the drug dose was taken by the mothers. In the milk, the median maximum concentration of olanzapine was 16 μg/liter at a median of 5.2 hours after the dose was taken by the mothers, while the median of the mean olanzapine concentrations in milk was 8.8 μg/liter. The median milk-to-plasma area-under-the-curve ratio for olanzapine was 0.38. The single milk-to-plasma ratio for subject G, measured 6 hours after the olanzapine dose was taken by the mother, was 0.75.
The median absolute infant dose (mean olanzapine concentration × 0.15 liter/kg/day) for the six patients with complete dose interval data was 1.32 μg/kg/day (25th–75th percentile range=0.74–1.71). The median relative infant olanzapine dose (weight-adjusted comparison with the maternal dose) in these six patients was 1.02%, with a 25th–75th percentile range of 0.93–1.12. For patient G, the absolute and relative infant doses, calculated from a measured milk concentration of 6 μg/liter, were 0.9 μg/kg/day and 1.13%, respectively.
Overall, olanzapine concentrations were below the limit of detection (varying from 1 μg/liter to 5 μg/liter) in the six infants from whom a plasma sample was taken at a median time of 6 hours (25th–75th percentile range=5.5–13.4 hours) after the last maternal dose was taken (Table 1).
Detailed medical examinations of infants B, C, E, and F showed no adverse effects. A Denver developmental examination of infant E indicated no developmental delay. A Griffiths developmental assessment conducted on infant D indicated a developmental age of 78% of her chronological age, but causality was difficult to assess because of recent maternal treatment with clonazepam, sertraline, valproate, thioridazine, and droperidol, as well as olanzapine. For logistic reasons, infant A was not assessed. Infant G was referred to this study because of drowsiness but was unaffected at the time of study after a halving of the maternal olanzapine dose to 5 mg/day 3 weeks earlier. Interviews with the infants’ mothers did not elicit any further problems. As a measure of infant progress, body weights on the study day were assessed with U.S. National Center for Health Statistics growth curves for children (22). On the study day, infants A, B, and E had reached a higher percentile curve than that indicated by their birth weights, but the weights of infants C and D, who were at the 97th percentile at birth, had dropped to the 90th and 50th percentiles, respectively. Infant F (who was 4 days old on the study day) had a weight that was above the 90th percentile at birth, despite olanzapine exposure during pregnancy. The weight of infant G was not available.
Discussion
The median and maximum relative infant doses of approximately 1.0% and 1.2% indicate that infant exposure to olanzapine through milk is much less than the notional cutoff of 10.0% that has been used to guide drug safety during breast-feeding (26). This low infant exposure was confirmed by failure to detect olanzapine in the plasma of six suckling infants and the lack of observed side effects in seven infants.
Of interest, our median milk-to-plasma ratio for olanzapine of 0.38 is identical to the theoretical milk-to-plasma ratio of 0.38 calculated by the method of Begg et al. (27). It is also similar to the mean of 0.46 measured by Croke et al. (16) from nine single-point milk and plasma concentrations measured in five patients.
The time at which maximum olanzapine concentrations occurred in milk was slightly later in the dosing interval than the corresponding maximum concentration in plasma. Mothers trying to minimize infant exposure may wish to avoid breast-feeding at peak milk concentrations of the drug (i.e., 5 hours postdose).
These data indicate that olanzapine may be considered relatively safe when mothers are breast-feeding healthy term infants. However, as always, risks and benefits should be considered before using any drug in breast-feeding mothers.
 |
Received Sept. 23, 2002; revision received Jan. 8, 2003; accepted Jan. 21, 2003. From the Department of Clinical Pharmacology, Christchurch Hospital; the Departments of Pharmacy, Neonatal Services, and Psychological Medicine, King Edward Memorial and Princess Margaret Hospitals, Subiaco, Western Australia; the Clinical Pharmacology and Toxicology Laboratory, Western Australian Centre for Pathology and Medical Research, Nedlands, Western Australia; the Mothers and Babies Service, Princess Margaret Hospital, Christchurch, New Zealand; and the Department of Pharmacology, University of Western Australia, Crawley, Western Australia. Address reprint requests to Ms. Gardiner, Department of Clinical Pharmacology, Christchurch Hospital, PB 4710 Christchurch, New Zealand; [email protected] (e-mail). Funded in part by the Women and Infants Research Foundation, Subiaco, Western Australia. The authors thank Hector Faulkiner, Michael Paech, Neville Butcher, Nigel Ford, and Nigel Orr for assistance with sample collection.
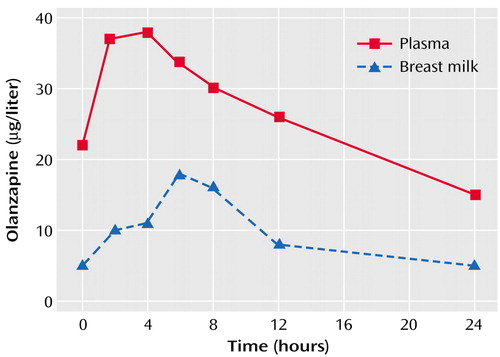
Figure 1. Plasma and Breast Milk Olanzapine Concentration of One Mother at Steady Statea
aEach drug concentration in milk was measured in aliquots in the middle interval of each expression/feeding period.
1. Zyprexa: Eli Lilly Product Information, in E-MIMS version 4.00.0602. St Leonards, Australia, MediMedia Australia, 2002. http://www.emims.net/login/about/about_home_fs.htmGoogle Scholar
2. Stephenson CM, Pilowsky LS: Psychopharmacology of olanzapine: a review. Br J Psychiatry Suppl 1999; 38:52–58Medline, Google Scholar
3. Caccia S: Biotransformation of post-clozapine antipsychotics: pharmacological implications. Clin Pharmacokinet 2000; 38:393–414Crossref, Medline, Google Scholar
4. Green B: Focus on olanzapine. Curr Med Res Opin 1999; 15:79–85Crossref, Medline, Google Scholar
5. Tollefson GD, Beasley CM Jr, Tran PV, Street JS, Krueger JA, Tamura RN, Graffeo KA, Thieme ME: Olanzapine versus haloperidol in the treatment of schizophrenia and schizoaffective and schizophreniform disorders: results of an international collaborative trial. Am J Psychiatry 1997; 154:457–465Link, Google Scholar
6. Kassahun K, Mattiuz E, Nyhart EJ, Obermeyer B, Gillespie T, Murphy A, Goodwin RM, Tupper D, Callaghan JT, Lemberger L: Disposition and biotransformation of the antipsychotic agent olanzapine in humans. Drug Metab Dispos 1997; 25:81–93Medline, Google Scholar
7. Fulton B, Goa KL: Olanzapine: a review of its pharmacological properties and therapeutic efficacy in the management of schizophrenia and related psychoses. Drugs 1997; 53:281–298Crossref, Medline, Google Scholar
8. Callaghan JT, Bergstrom RF, Ptak LR, Beasley CM: Olanzapine: pharmacokinetic and pharmacodynamic profile. Clin Pharmacokinet 1999; 37:177–193Crossref, Medline, Google Scholar
9. Kelly DL, Conley RR, Tamminga CA: Differential olanzapine plasma concentrations by sex in a fixed-dose study. Schizophr Res 1999; 40:101–104Crossref, Medline, Google Scholar
10. Olesen OV, Linnet K: Olanzapine serum concentrations in psychiatric patients given standard doses: the influence of co-medication. Ther Drug Monit 1999; 21:87–90Crossref, Medline, Google Scholar
11. Pritchard DB, Harris B: Aspects of perinatal psychiatric illness. Br J Psychiatry 1996; 169:555–562Crossref, Medline, Google Scholar
12. Brockington IF, Cernik KF, Schofield EM, Downing AR, Francis AF, Keelan C: Puerperal psychosis: phenomena and diagnosis. Arch Gen Psychiatry 1981; 38:829–833Crossref, Medline, Google Scholar
13. Agrawal P, Bhatia MS, Malik SC: Post partum psychosis: a clinical study. Int J Soc Psychiatry 1997; 43:217–222Crossref, Medline, Google Scholar
14. Bhana N, Foster RH, Olney R, Plosker GL: Olanzapine: an updated review of its use in the management of schizophrenia. Drugs 2001; 61:111–161Crossref, Medline, Google Scholar
15. Yatham LN: The role of novel antipsychotics in bipolar disorders. J Clin Psychiatry 2002; 63(suppl 3):10–14Medline, Google Scholar
16. Croke S, Buist A, Hackett LP, Ilett KF, Norman T, Burrows GD: Olanzapine excretion in human breast milk: estimation of infant exposure. Int J Neuropsychopharmacol 2002; 5:243–247Crossref, Medline, Google Scholar
17. Goldstein DJ, Corbin LA, Fung MC: Olanzapine-exposed pregnancies and lactation: early experience. J Clin Psychopharmacol 2000; 20:399–403Crossref, Medline, Google Scholar
18. Kirchheiner J, Berghöfer A, Bolk-Weischedel D: Healthy outcome under olanzapine treatment in a pregnant woman. Pharmacopsychiatry 2000; 33:78–80Crossref, Medline, Google Scholar
19. Rossiter EJ: The use of developmental screening and assessment instruments by paediatricians in Australia. J Paediatr Child Health 1993; 29:357–359Crossref, Medline, Google Scholar
20. Frankenburg WK, Dodds JB: The Denver Development Screening Test. J Pediatr 1967; 71:181–191Crossref, Medline, Google Scholar
21. Griffiths R: The Abilities of Babies: A Study in Mental Development. Amersham, Bucks, UK, Association for Research in Child and Infant Development, 1976Google Scholar
22. Hamill PV, Drizd TA, Johnson CL, Reed RB, Roche AF: NCHS Growth Curves for Children: Birth-18 Years: United States. Vital Health Stat 11 1977; 165:1–74Google Scholar
23. Dusci LJ, Hackett LP, Fellows LM, Ilett KF: Determination of olanzapine in plasma by high-performance liquid chromatography using ultraviolet absorbance detection. J Chromatogr B Analyt Technol Biomed Life Sci 2002; 773:191–197Crossref, Medline, Google Scholar
24. Thomann P: Non-compartmental analysis methods manual, in TopFit 2.0 Pharmacokinetic and Pharmacodynamic Data Analysis System for the PC. Edited by Heinzel G, Woloszcak R, Thomann P. Stuttgart, Gustav Fischer, 1993, pp 5–66Google Scholar
25. Rampono J, Kristensen JH, Hackett LP, Paech M, Kohan R, Ilett KF: Citalopram and demethylcitalopram in human milk: distribution, excretion and effects in breast-fed infants. Br J Clin Pharmacol 2000; 50:263–268Crossref, Medline, Google Scholar
26. Bennett PN: Use of the monographs on drugs, in Drugs and Human Lactation. Edited by Bennett PN. Amsterdam, Elsevier, 1996, pp 67–74Google Scholar
27. Begg EJ, Atkinson HC, Duffull SB: Prospective evaluation of a model for the prediction of milk: plasma drug concentrations from physicochemical characteristics. Br J Clin Pharmacol 1992; 33:501–505Crossref, Medline, Google Scholar



