Effect Size of Symptom Status in Withdrawal of Typical Antipsychotics and Subsequent Clozapine Treatment in Patients With Treatment-Resistant Schizophrenia
Abstract
OBJECTIVE: In light of the efficacy of newer antipsychotic agents and the possibility that drug withdrawal may negatively affect subsequent drug response, concern has arisen that the use of placebo in schizophrenia research may be unethical. This study examines the effect size of symptom exacerbation during drug washout with placebo and the effects of drug washout on the efficacy of subsequent drug treatment. METHOD: Fifty patients with treatment-resistant schizophrenia hospitalized on a research unit participated in a double-blind longitudinal study of the effects of drug washout after chronic treatment with a typical antipsychotic and before prospective treatment with clozapine. Brief Psychiatric Rating Scale (BPRS) scores were analyzed to examine drug effects and effect sizes for baseline treatment with a typical antipsychotic (>6 months treatment), drug washout with placebo (mean=34 days), early treatment with clozapine (mean=42 days, mean dose=345.0 mg/day), and optimal clozapine treatment (mean=83 days, mean dose=450.5 mg/day). RESULTS: Patients’ BPRS total, positive, and negative symptom scores significantly increased during placebo washout, compared with baseline treatment, and significantly decreased with administration of clozapine, compared with placebo washout and baseline treatment. However, 30% of patients showed some symptom improvement during placebo washout. The effect sizes for the BPRS total score were 0.63 for baseline treatment versus placebo washout, 1.10 for optimal clozapine treatment versus placebo washout, and 0.82 for optimal clozapine treatment versus baseline treatment. CONCLUSIONS: Symptom exacerbation induced by drug withdrawal in patients with treatment-resistant schizophrenia did not impede subsequent responsiveness to clozapine. The effect size for clozapine, compared with typical antipsychotics, suggests that the drug-washout longitudinal design is useful for establishing a drug-free baseline and for investigating drug response, while requiring relatively few subjects.
Two trends of the past decade have had a major influence on clinical trials of new drugs to treat schizophrenia: 1) increasing concerns about whether the use of drug washout periods is ethical (1–3) and 2) the transition to use of “atypical” antipsychotic drugs in clinical practice (4). It has become widely assumed, although far from proven (5), that atypical antipsychotics as a group, in addition to having more favorable extrapyramidal side effect profiles, show superior efficacy, compared with traditional antipsychotic drugs. Much of this belief is based on the demonstrated superiority of clozapine in comparison with traditional antipsychotic drugs and the comparability of other atypical antipsychotics to clozapine in subsequent clinical studies (5, 6). The superiority of atypical antipsychotics, other than clozapine, in direct comparison to traditional agents in treating the core symptoms of schizophrenia is only modestly supported by clinical trials (5).
It has been hypothesized that antipsychotic discontinuation may negatively influence pathophysiological mechanisms and clinical outcome in schizophrenia (7), although this thesis remains controversial (8–12). Whereas it is untenable to argue that psychotic exacerbation brought about by the discontinuation of antipsychotic drug treatment is experientially benign, the effects of drug withdrawal on outcome or on subsequent response to drug treatment remain to be more clearly elucidated. Moreover, as drug development proceeds to include potential antipsychotic drugs with unproven underlying mechanisms, the utilization of drug-free periods will be needed for critical proof-of-concept experiments (13). Drug washout periods also remain important for establishing levels of symptoms and biological parameters characteristic of an individual’s illness without the “contamination” of existing drug treatment (9–11). Indeed, the definition of drug response as a change from a drug-free condition has become increasingly important with the development of pharmacogenetic studies in which data on candidate genes associated with specific brain processes are used to predict the outcome of drug treatment (14).
Clinical trials whose goal is to lead to approval of a drug by the U.S. Food and Drug Administration (15) employ a parallel design in which one arm may include a placebo treatment. These studies also characteristically rely on truncated placebo arms (i.e., not all patients complete the treatment period) and often use the last-observation-carried-forward method, in which the last ratings before dropout are carried forward to the endpoint (16, 17). Patient dropout, however, may precede psychotic symptom exacerbation (18). For this reason, it is not surprising that placebo arms in these studies often show modest or no symptom exacerbation (16), despite the prevailing understanding that patients removed from antipsychotic treatment worsen substantially.
In this paper we report data from studies involving patients with treatment-resistant schizophrenia treated on an intramural NIMH research ward. The studies examined the behavioral effects of drug washout and subsequent treatment with clozapine and were carried out under double-blind treatment conditions. Behavioral change after discontinuation of antipsychotic treatment was quantified, and the hypothesis that symptom exacerbation during washout negatively affects future drug response was tested.
Method
Patients
Fifty psychiatric inpatients who were hospitalized on the 4-East Clinical Center Research Ward at the National Institutes of Health (19) and who met the DSM-III-R criteria for schizophrenia and the criteria of Kane et al. (20) for treatment resistance participated in a prospective evaluation of antipsychotic drug withdrawal and subsequent treatment with the atypical antipsychotic, clozapine. All patients provided written informed consent before participation in the protocol. The consent process included the presentation of the inherent risks of antipsychotic drug withdrawal, including possible worsening of symptoms, as well as of the potential benefits of clozapine.
Of the 50 patients, 35 were male (32 were Caucasian, two were African American, and one was Asian American) and 15 were female (14 were Caucasian, and one was African American). Their mean age was 31.6 years (SD=7.3) at the time of protocol participation and 20.5 years (SD=5.9) at illness onset. Their mean number of prior hospitalizations was 5.7 (SD=5.1), and their mean number of months of hospitalization before the index research hospitalization was 10.0 (SD=12.3). Twenty-two percent of the patients had made at least one suicide attempt before the index hospitalization.
Study Design
The longitudinal study was carried out by using double-blind methods throughout. All patients had been chronically treated with typical antipsychotic agents for 6 months or longer before admission to the study. The study protocol had four consecutive treatment phases: 1) baseline treatment with a typical antipsychotic, 2) drug washout with placebo, 3) early treatment with clozapine, and 4) optimal treatment with clozapine. All patients completed all phases of the study. This design was described in previous reports on subgroups of these patients who participated in studies assessing clinical and biological measures of clozapine treatment, including metabolite levels (21), response to a serotonin agonist (22), psychophysiology (23), dopamine D2 receptor occupancy (24), and regional brain metabolic effects (25).
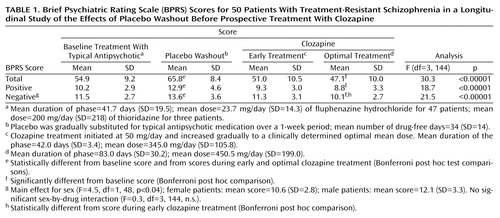
During the baseline phase, chronic antipsychotic treatment was converted to fluphenazine hydrochloride for 47 patients (mean dose=23.7 mg/day, SD=14.3); three patients were treated with thioridazine because of persistent extrapyramidal side effects (mean dose=200 mg/day, SD=218). Placebo substitution was carried out in parallel with a gradual reduction in the dose of typical antipsychotic medication so that the patients were treated with placebo alone at the end of 1 week after the initiation of the decrease in antipsychotic drug dose. The mean length of baseline treatment with a typical antipsychotic before placebo substitution was 41.7 days (SD=19.5). The time to initiation of placebo substitution was not fixed but rather varied individually to ensure availability of clinical resources to manage a drug-free patient and to obtain stable symptom ratings while the patient was taking fluphenazine. The placebo washout period was designed to last at least 4 weeks for each patient. Thirty percent of the patients (15 of 50), however, were drug free less than 28 days (minimum=7 days) because of clinical considerations. Overall, the mean number of drug-free days was 34 (SD=14). In some cases patients continued to receive placebo for longer periods on the basis of clinical considerations such as lack of relapse and clinical stability.
The placebo washout period was followed by clozapine treatment initiated at 50 mg/day and increased gradually as tolerated over 2–3 weeks to a clinically determined optimal mean dose. Two time points during clozapine treatment were chosen for determining the relative effect sizes of clozapine in comparison to placebo washout and to chronic treatment with a typical antipsychotic: 1) early clozapine treatment (mean duration of treatment=42.0 days [SD=3.4]; mean dose=345.0 mg/day [SD=105.8]) and 2) optimal clozapine treatment (mean duration of treatment=83.0 days [SD=30.2]; mean dose=450.5 mg/day [SD=199.0]).
Behavioral Ratings
The Brief Psychiatric Rating Scale (BPRS) (26) was administered weekly by psychiatrists who were blind to the patients’ medication status throughout the study. BPRS total and positive and negative symptom scores were obtained. The BPRS positive symptom cluster consists of items assessing disorganized speech and thought disorder, hallucinations, delusions, and distractibility. The negative symptom cluster includes items assessing emotional withdrawal, psychomotor retardation, blunted affect, and loss of social function.
Statistical Analysis
Each patient had data for four rating periods: the final week of baseline treatment with a typical antipsychotic, the last week of the placebo washout period, the last week of early clozapine treatment, and the last week of optimal clozapine treatment. Statistical analysis of BPRS scores was done with repeated-measures analysis of variance (ANOVA) and the Greenhouse-Geisser correction, with the factors of drug treatment (time) and sex. Post hoc analysis was done with the Bonferroni post hoc t test by using the appropriate mean square error from the repeated-measures ANOVA to establish a protected 1% type I error. Effect size (“d”) was calculated by using the method of Cohen (27) for paired samples. An effect size of 0.20 was considered small, 0.50 was medium, and 0.80 was large.
Results
Group Data
As shown in Table 1 and Table 2, drug withdrawal (placebo substitution) resulted in statistically significant increases in BPRS total and positive and negative symptom scores across treatment conditions, with larger than medium effect sizes. For all BPRS scores, clozapine treatment produced a statistically significant reduction in symptoms that progressed from early to optimal treatment. Statistically significant reductions in all symptom ratings, compared to ratings during placebo washout, were found for both clozapine treatment conditions, although a statistically significant reduction, compared with baseline treatment with a typical antipsychotic, was found only during the optimal clozapine period. In addition, negative symptom scores showed a statistically significant reduction from early to optimal clozapine treatment.
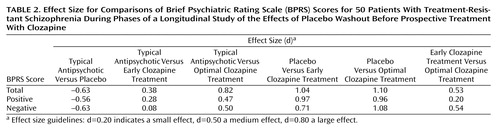
As Table 2 shows, placebo washout was associated with modest effect sizes for BPRS total and positive and negative symptom scores, whereas considerably larger effect sizes were seen for both early and optimal clozapine treatment, compared with placebo washout. For the BPRS total score, the effect size for optimal clozapine treatment, compared to baseline treatment with a typical antipsychotic, was greater than the effect size for placebo, compared with baseline treatment.
Individual Patient Data
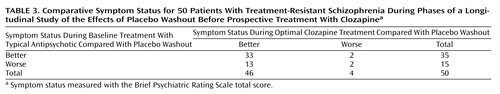
Table 3 summarizes outcomes for the patients grouped by whether they did better or worse while receiving baseline treatment with a typical antipsychotic, placebo, or optimal clozapine treatment. Whereas placebo was, overall, associated with a statistically significant increase in the BPRS total score, 30% of the patients (15 of 50) did better (had lower scores) while receiving placebo than while receiving baseline treatment. Although doing better while receiving baseline treatment, compared with placebo, was predictive of doing better while receiving optimal clozapine treatment, compared with placebo (33 of 35 patients), doing worse while receiving baseline treatment was not predictive of doing worse while receiving optimal clozapine treatment. Rather, patients who did worse at baseline, compared with the placebo washout period, did better while receiving optimal clozapine treatment (13 of 15 patients) (McNemar χ2=6.67, df=1, p<0.01).
After discontinuation of baseline treatment with typical antipsychotics, 60% of the patients (30 of 50) showed a marked worsening of symptoms (≥10% increase in the BPRS total score), 14% (7 of 50) showed a marked improvement (≥10% decrease in the BPRS total score), and 26% (13 of 50) showed moderate symptom change (less than a 10% increase or decrease in the BPRS total score). The patients who showed a marked worsening during drug withdrawal overall had a mean increase in symptom ratings of >40%, and those with marked improvement during drug withdrawal had a mean decrease in symptom ratings of >20%.
Patients who improved during the placebo washout, compared with baseline treatment with a typical antipsychotic, showed greater improvement during optimal clozapine treatment, compared with baseline treatment, than patients who worsened during placebo washout (Figure 1).
Discussion
This study demonstrates that patients with schizophrenia who meet the criteria for treatment resistance (20) show symptom exacerbation after the discontinuation of chronic treatment with a typical antipsychotic. However, the clinical deterioration induced by drug withdrawal does not appear to negatively affect subsequent response to treatment with clozapine. Group mean BPRS scores showed that clozapine treatment resulted in a significant improvement in symptoms, compared with both placebo and baseline treatment with a typical antipsychotic. Individual patient data revealed that although a majority (60%) of patients showed marked deterioration (≥10% worsening) after discontinuation of baseline drug treatment, some patients actually had a decrease in symptoms after drug discontinuation. It is interesting to note that patients whose symptoms decreased the most during drug washout showed marked further improvement during clozapine treatment, suggesting that, at least for some patients, responsiveness to typical antipsychotic treatment should not necessarily be equated to responsiveness to clozapine. It should be considered, however, that the study had no control condition for clozapine response in patients without a prior drug withdrawal period, raising the possibility that drug withdrawal compromised the degree of clozapine-induced improvement that might otherwise have been seen. Moreover, drug withdrawal could have different effects when carried out at different stages of the illness, for example, after a first break or during recovery from an acute psychotic exacerbation of chronic illness.
The sensitivity of patients with treatment-resistant schizophrenia to drug discontinuation suggests that the concept of “treatment resistance” is far from absolute. The clinical characteristics of the patients in our study were typical of seriously ill patients with schizophrenia (19), although the selection criteria for NIMH research studies minimize the inclusion of patients at high risk for violence or severe noncompliance. The effect size of 0.63 for drug discontinuation for the overall group for the BPRS total score was between the accepted levels of medium (d=0.50) and large (d=0.80), although the exclusion of more uncooperative patients, as well as the ready availability of intervention if clinical deterioration was detected, may have led to underestimation of the actual effect size of drug withdrawal. Although the design of this study addressed response to traditional antipsychotic treatment through the “window” of drug discontinuation, it did emphasize that the identification of an “inadequate” response to prior trials of typical antipsychotics is relative. Clinical studies whose patient selection criteria may exclude patients with “treatment-resistant” disorders in order to reduce the number of nonresponsive patients in the clinical trial population in which a new drug is tested may be unwisely excluding a patient group that could provide valuable data. Measurement of behavioral change after discontinuation might be the best way to identify patients with inherent responsiveness to traditional antipsychotic treatment.
The enhanced therapeutic effectiveness of clozapine in seriously ill patients with schizophrenia was again demonstrated in this study. Statistically significant improvement during optimal clozapine treatment, compared to baseline treatment with a typical antipsychotic, for BPRS total and positive and negative symptom scores was clearly demonstrated. Early clozapine treatment, however, while associated with statistically significant improvement compared with placebo, was not significantly different in effectiveness from baseline treatment with a typical antipsychotic. The greater effectiveness of optimal clozapine treatment than early clozapine treatment, in relation to baseline treatment with a typical antipsychotic, would appear to be related to the interaction of time (longer treatment) and dose (higher dose). It is interesting to note that the negative symptom score was the only treatment variable that showed statistically significant superiority of optimal clozapine treatment, compared with early clozapine treatment, supporting the need for prolonged treatment with clozapine to elicit its full effects on some treatment complexes (28).
Effect size provides a measure by which variable difference is “standardized,” i.e., it is expressed in relation to the variation (standard deviation). For this reason, effect size is well suited for comparison of results across studies (29). In this study, very large effect sizes for BPRS total scores were found for both early and optimal clozapine treatment, compared with placebo (d=1.04 and d=1.10, respectively). Most studies comparing clozapine (or other atypical antipsychotics) with typical antipsychotics have been carried out by using a randomized, multiarm parallel design in which one group of patients is treated with a typical antipsychotic and another group is treated with clozapine (5). The longitudinal design used here enabled the assessment of drug-free symptom levels, a critical component for biological research and rater consistency; this design enhances power while including a smaller number of subjects (30, 31). It is interesting to note that the effect size of 0.82 for the BPRS total score for optimal clozapine treatment, compared with baseline treatment with a typical antipsychotic, is nearly identical to the effect size of 0.81 calculated by Chakos et al. (5) for the study by Kane et al. (20) comparing clozapine and chlorpromazine in a parallel design. In contrast to the large effect size for the BPRS total score, medium effect sizes were found for positive (d=0.47) and negative (d=0.50) symptom scores for optimal clozapine treatment, compared to baseline treatment with a typical antipsychotic, lending some support for the idea that these symptom complexes may not be the most sensitive to the therapeutic effects of clozapine (32).
Our study findings also suggest that a placebo-controlled, multiarm, parallel-design clinical trial with truncated treatment arms (e.g., last observation carried forward) may substantially underestimate behavior indicating clinical deterioration during placebo treatment. For example, examination of results for BPRS total scores from the placebo arm of studies by Marder and Meilbach (33) and Beasely et al. (34)—clinical trials that examined the effects of risperidone and olanzapine, respectively, compared to placebo—showed a mean change of 3% and an effect size of 0.15 in the former and a mean change of 8% and an effect size of 0.18 in the latter. In both studies, the mean change in scores and the effect size are substantially smaller than the mean change of 20% and the effect size of 0.63 found in our study. However, for the 58% of the patients in our study who showed a marked symptom exacerbation during drug washout (≥10% increase in symptom ratings), symptom ratings worsened by a mean of 43% during placebo washout, compared with baseline treatment with a typical antipsychotic, and a very large effect size of 1.5 was found. Thus, underestimation of placebo response may be particularly pronounced in studies that include patients with schizophrenia that is not treatment resistant. Relapse rate—a variable that is difficult to connect to symptom ratings—after neuroleptic withdrawal has been shown to increase over time, with a 50% relapse rate occurring at approximately 6 months after neuroleptic withdrawal and with time to relapse influenced by the rate of drug discontinuation (35, 36).
Our findings provide some support for the idea of “treatment resistance” to conventional antipsychotic medication insofar as 30% of the patients improved during the drug washout. It is interesting to note that the patients who improved during the drug washout showed further improvement during clozapine treatment. Although these findings are difficult to interpret, they give some credence to the possibility that some patients have differential responsiveness to traditional antipsychotic treatment and to clozapine.
In summary, our study provides data regarding the effect size of drug discontinuation in seriously ill patients with schizophrenia in a research ward setting. The concept of treatment resistance is a relative one, and patients who meet the criteria for treatment-resistant disorders are not necessarily insensitive to drug treatment. Our study also demonstrates that symptom exacerbation induced by drug withdrawal does not appear to negatively affect the effectiveness of subsequent treatment with clozapine. The study findings highlight the need for designers of multiarm, parallel-design clinical trials to use caution when including a truncated placebo arm as a comparator to antipsychotic treatment, particularly if the study involves proof-of-concept of a new mechanism. Finally, in this study, clozapine again displayed an enhanced therapeutic profile, compared to traditional antipsychotic treatment, with the greatest effectiveness for clozapine seen over time (>6 weeks) at a dose of approximately 450 mg/day.
 |
 |
 |
Received July 23, 2002; revision received Dec. 27, 2002; accepted Jan. 7, 2003. From Gabriel Pharma. Address reprint requests to Dr. Pickar, Gabriel Pharma, 6500 Seven Locks Rd., Cabin John, MD 20818; [email protected] (e-mail). Supported in part by NIMH Small Business Innovation Research grant MH-67351-01. The authors thank the study patients’ physicians and nurses for providing expert clinical care and the study patients for their participation.
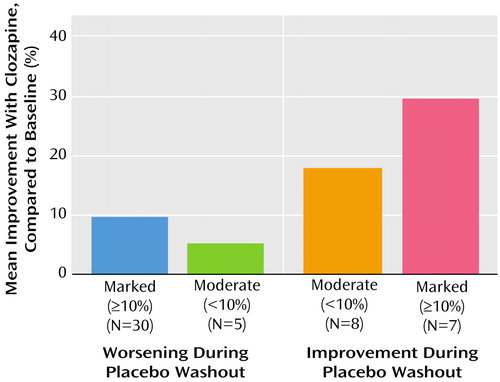
Figure 1. Improvement During Optimal Clozapine Treatment, Compared to Baseline Treatment With a Typical Antipsychotic, for 50 Patients With Treatment-Resistant Schizophrenia Grouped by Change in Symptom Status During Placebo Washouta
aSymptom status measured with the Brief Psychiatric Rating Scale total score. The largest mean improvement during optimal clozapine treatment occurred among patients who improved during the placebo washout period and experienced further improvement during optimal clozapine treatment (F=3.7, df=3, 46, p<0.02).
1. Rothman KJ, Michels KB: The continuing unethical use of placebo controls. N Engl J Med 1994; 331:394-398Crossref, Medline, Google Scholar
2. Hyman SE, Shore D: An NIMH perspective on the use of placebos. Biol Psychiatry 2000; 47:689-691Crossref, Medline, Google Scholar
3. Pinals DA, Applebaum PS: Ethical aspects of neuropsychiatric research with human subjects, in Neuropsychopharmacology: The Fifth Generation of Progress. Edited by Davis KL, Charney D, Coyle JT, Nemeroff C. Philadelphia, Lippincott Williams & Wilkins, 2002, pp 475-483Google Scholar
4. Beers MH, Berkow R (eds): Merck Manual of Diagnosis and Therapy, 17th ed. Whitehouse Station, NJ, Merck Research Laboratories, 1999, pp 1563-1571Google Scholar
5. Chakos M, Lieberman J, Hoffman E, Bradford D, Sheitman B: Effectiveness of second-generation antipsychotics in patients with treatment-resistant schizophrenia: a review and meta-analysis of randomized trials. Am J Psychiatry 2001; 158:518-526Link, Google Scholar
6. Miyamoto S, Duncan GE, Goff DC, Lieberman JA: Therapeutics in schizophrenia, in Neuropsychopharmacology: The Fifth Generation of Progress. Edited by Davis KL, Charney D, Coyle JT, Nemeroff C. Philadelphia, Lippincott Williams & Wilkins, 2002, pp 775-807Google Scholar
7. Wyatt RJ: Risks of withdrawing antipsychotic medications. Arch Gen Psychiatry 1995; 52:205-208Crossref, Medline, Google Scholar
8. Curson DA, Hirsch SR, Platt SD: Does short term placebo treatment of chronic schizophrenia produce long term harm? Br Med J (Clin Res Ed) 1986; 293:726-728Crossref, Medline, Google Scholar
9. Carpenter WT, Tamminga CA: Why neuroleptic withdrawal in schizophrenia? Arch Gen Psychiatry 1995; 52:192-193Crossref, Medline, Google Scholar
10. Baldessarini RJ, Viguera AC: Neuroleptic withdrawal in schizophrenic patients. Arch Gen Psychiatry 1995; 52:173-188Crossref, Medline, Google Scholar
11. Jeste DV, Palmer BS, Harris MJ: Neuroleptic discontinuation in clinical and research settings: scientific issues and ethical dilemmas. Biol Psychiatry 1999; 46:1050-1059Crossref, Medline, Google Scholar
12. Lieberman JA, Perkins D, Belger A, Chakos M, Jarskog F, Boteva K, Gilmore J: The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry 2001; 50:884-897Crossref, Medline, Google Scholar
13. Wong DF, Potter WZ, Brasic JR: Proof of concept: functional models for drug development in humans, in Psychopharmacology: The Fifth Generation of Progress. Edited by Davis KL, Charney D, Coyle JT, Nemeroff C. Philadelphia, Lippincott Williams & Wilkins, 2002, pp 457-473Google Scholar
14. Pickar D, Rubinow K: Pharmacogenomics of psychiatric disorders. Trends Pharmacol Sci 2001; 22:75-83Crossref, Medline, Google Scholar
15. Leber P: The use of placebo control groups in the assessment of psychiatric drugs: an historical context. Biol Psychiatry 2000; 47:699-706Crossref, Medline, Google Scholar
16. Lavori PW: Placebo control groups in randomized treatment trials: a statistician’s perspective. Biol Psychiatry 2000; 47:717-723Crossref, Medline, Google Scholar
17. Klein DF, Thase M, Endicott J, Adler L, Glick I, Kalali A, Leventer S, Mattes J, Ross P, Bystritsky A: Improving clinical trials. Arch Gen Psychiatry 2002; 59:272-278Crossref, Medline, Google Scholar
18. Dencker SJ, Malm U, Lepp M: Schizophrenic relapse after drug withdrawal is predictable. Acta Psychiatr Scand 1986; 73:181-185Crossref, Medline, Google Scholar
19. Schreiber JL, Breier A, Pickar D: Characteristics of patients selected for treatment on a schizophrenia research ward. Hosp Community Psychiatry 1990; 41:441-443Abstract, Google Scholar
20. Kane J, Honigfeld G, Singer J, Meltzer H (Clozaril Collaborative Study Group): Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988; 45:789-796Crossref, Medline, Google Scholar
21. Pickar D, Owen RR, Litman R, Konicki E, Guiterez R, Rapaport MH: Clinical and biological response to clozapine in patients with schizophrenia. Arch Gen Psychiatry 1992; 49:345-353Crossref, Medline, Google Scholar
22. Owen RR Jr, Gutierrez-Esteinou R, Hsiao J, Hadd K, Benkelfat C, Lawlor BA, Murphy DL, Pickar D: Effects of clozapine and fluphenazine treatment on responses to m-chlorophenylpiperazine infusions in schizophrenia. Arch Gen Psychiatry 1993; 50:636-644Crossref, Medline, Google Scholar
23. Zahn TP, Pickar D: Autonomic effects of clozapine in schizophrenia: comparison with placebo and fluphenazine. Biol Psychiatry 1993; 34:3-12Crossref, Medline, Google Scholar
24. Pickar D, Su T-P, Weinberger DR, Coppola R, Malhotra AK, Knable MB, Lee KS, Gorey J, Bartko JJ, Breier A, Hsiao J: Individual variation in D2 dopamine receptor occupancy in clozapine-treated patients. Am J Psychiatry 1996; 153:1571-1578Link, Google Scholar
25. Cohen RM, Nordahl TE, Semple WE, Andreasen P, Litman RE, Pickar D: The brain metabolic patterns of clozapine and fluphenazine treated patients with schizophrenia during a continuous performance task. Arch Gen Psychiatry 1997; 54:481-486Crossref, Medline, Google Scholar
26. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertaining and scaling. Psychopharmacol Bull 1988; 24:97-99Google Scholar
27. Cohen J: Statistical Power Analysis for the Behavioral Sciences, revised ed. New York, Academic Press, 1977Google Scholar
28. Conley RR, Kelly DL: Management of treatment resistance in schizophrenia. Biol Psychiatry 2001; 50:898-911Crossref, Medline, Google Scholar
29. Rosenthal R: Meta Analytic Procedures for Social Research, revised ed. New York, Sage Publications, 1991Google Scholar
30. Kane JM: Issues in clinical trials designs, in Neuropsychopharmacology: The Fifth Generation of Progress. Edited by Davis KL, Charney D, Coyle JT, Nemeroff C. Philadelphia, Lippincott Williams & Wilkins, 2002, pp 537-546Google Scholar
31. Perkins DO, Wyatt RJ, Bartko JJ: Penny-wise and pound-foolish: the impact of measurement error on sample size requirements in clinical trials. Biol Psychiatry 2000; 47:762-766Crossref, Medline, Google Scholar
32. McMahon RP, Kelly DL, Kreyenbuhl J, Kirkpatrick B, Love RC, Conley RR: Novel factor-based symptom scores in treatment resistant schizophrenia: implications for clinical trials. Neuropsychopharmacology 2002; 26:537-545Crossref, Medline, Google Scholar
33. Marder SR, Meilbach RC: Risperidone in the treatment of schizophrenia. Am J Psychiatry 1994; 151:825-835Link, Google Scholar
34. Beasley CM, Tollefson G, Tran P, Satterliee W, Sanger T, Hamilton S: Olanzapine versus placebo and haloperidol. Neuropsychopharmacology 1996; 14:111-123Crossref, Medline, Google Scholar
35. Jeste DV, Palmer BS, Harris MJ: Neuroleptic discontinuation in clinical and research settings: scientific issues and ethical dilemmas. Biol Psychiatry 1999; 46:1050-1059Crossref, Medline, Google Scholar
36. Gilbert PL, Harris MJ, McAdams A, Jeste DV: Neuroleptic withdrawal in schizophrenia: a review of the literature. Arch Gen Psychiatry 1995; 52:173-188Crossref, Medline, Google Scholar



