Deficits in Gain of Smooth Pursuit Eye Movements in Schizophrenia and Affective Disorder Patients and Their Unaffected Relatives
Abstract
OBJECTIVE: Disturbance of smooth pursuit eye movements has been discussed as marking a putative endophenotype closely associated with the genetic basis of schizophrenia. Previous studies are not conclusive in regard to the specificity of this marker. Therefore, oculomotor pursuit was evaluated in unaffected family members of index probands diagnosed as having either schizophrenia or affective disorders. METHOD: A series of eye tracking tasks were performed by 54 patients with schizophrenia or schizoaffective disorder, 46 patients with an affective disorder, 43 unaffected first-degree relatives of the schizophrenia patients, 36 unaffected first-degree relatives of the affective disorder patients, and 84 healthy comparison subjects. The gain, which relates the velocity of the eye movement to the velocity of the target, was determined to index the intactness of the oculomotor pursuit system. RESULTS: Mean pursuit gain was significantly lower in the schizophrenia and affective disorder patients than in the healthy comparison subjects. Moreover, the relatives of both the schizophrenia and affective disorder patients showed significant gain deficits of about one-half the size of those observed in the patients. CONCLUSIONS: Gain deficits are present in psychotic patients and in their unaffected biological relatives. This finding supports a genetic origin of eye tracking disturbances in major psychotic disorders. There is no evidence for diagnostic or familial specificity. The weak sensitivity of the marker suggests that it refers to a nonnecessary genetic factor in schizophrenic and affective disorders.
Abnormalities of smooth pursuit eye movements in schizophrenia patients and their first-degree relatives have been frequently documented. This gave rise to the supposition that this deficit might serve as a phenotypic marker for the genetic liability for schizophrenia (1, 2). Such a marker could be highly important for molecular genetic studies because the clinical phenotype of schizophrenia has questionable validity for the genotype of the disorder. Case identification in genetic studies, which is based solely on psychopathology, may be incorrect as a consequence of either pleiotropic effects, i.e., expressions of the schizophrenic genotype not covered by the current clinical classification systems, or phenocopies, i.e., cases clinically diagnosed as schizophrenia that do not possess the schizophrenia genotype. A valid marker would overcome this problem in part by being more closely associated with the biological core of the disorder than with the actual clinical diagnosis (3, 4).
Dysfunction of smooth pursuit eye movements fulfills several of the criteria for a phenotypic marker of genetic liability to schizophrenia (5). Most important, it has been proven in twin studies to be highly heritable (6, 7). In addition, deficits in smooth pursuit eye movements have been shown to aggregate in families with members diagnosed as having schizophrenia. First-degree relatives of affected probands showed higher prevalences than members of unaffected families (8–15). Another criterion for a trait to qualify as a genetic liability marker is its specificity for a disorder. In this regard, however, previous findings have been less clear. Several studies (16–18) demonstrated deficits in smooth pursuit eye movements in bipolar disorder patients similar to those in schizophrenia patients. Patients with unipolar depression also showed pursuit deficits (12, 19). Normal performance in affective disorder patients was found in a minority of studies (8, 20, 21), and only one study (22) used the gain as a performance measure. Using step-ramp stimuli, Sweeney et al. (23) found lower than normal gain both in patients with acute schizophrenia and in patients with mood disorders. Treatment with lithium has been discussed as a possible explanation for the deficits in smooth pursuit eye movements repeatedly found in affective disorder patients, thereby masking the specificity (17, 20, 24). A careful study by Gooding et al. (25), however, did not show effects of lithium in first-episode patients with any of several measures used; there was no significant difference either between treatment groups or within the lithium group over time. In the light of these findings, the specificity for schizophrenia of deficits in smooth pursuit eye movements remains controversial.
A general problem for the interpretation of differences between patient groups is the fact that psychopathological status and medication may interfere with performance. Therefore, a powerful strategy to support the specificity of a marker is the investigation of unaffected first-degree relatives of patients. The marker should be present in some of the relatives of the schizophrenia patients but not in the relatives of patients with other diagnoses. Studies using this approach have yielded mixed results (8, 9, 12, 26). Thus, convincing evidence of familial specificity has not been produced so far. Few family studies have used specific measures for the oculomotor systems according to neuro-ophthalmic standards. Such measures are capable of distinguishing between pursuit deficits and saccadic intrusions, and therefore they allow conclusions to be made about whether or not a particular system is dysfunctional (1, 27, 28). In the majority of studies (10, 13, 15) that determined the mean pursuit gain, but not in all (14), it was found to be less than normal in relatives of patients with schizophrenia, indicating a distinct abnormality of the pursuit system. We do not know of any reports on gain measures in relatives of probands with affective disorder.
Therefore, the purposes of our study were 1) to replicate earlier findings of lower gain in patients diagnosed as having schizophrenia than in healthy subjects, 2) to test the hypothesis that lower gain can also be found in affective disorder patients, 3) to find further evidence for the hypothesis that first-degree relatives of schizophrenia patients also show lower gain than healthy subjects, and 4) to test familial specificity by comparing these relatives with first-degree relatives of affective disorder patients and with healthy comparison subjects.
Method
Participants
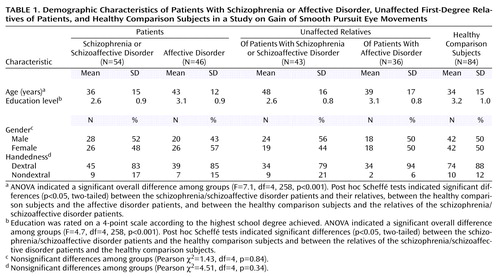
One hundred psychiatric patients, 79 unaffected first-degree relatives of these patients, and 84 healthy subjects participated in this study. Their ages ranged from 18 to 70 years. After complete description of the study, each participant gave written informed consent. Demographic characteristics of the subgroups are presented in Table 1.
Psychiatric patients
As index probands, inpatients from three psychiatric hospitals (University of Munich, Psychiatric Hospital of Salzburg, and Psychiatric State Hospital of Kaufbeuren) were recruited and screened for the study by the psychiatrists responsible for their treatment. The inclusion criteria were then reevaluated by research psychiatrists using standardized assessment methods. Patients were included if they had a confirmed diagnosis of schizophrenia, schizoaffective disorder, or affective disorder (major depression or bipolar disorder), if they had a positive family history for this disorder (at least one first- or second-degree relative with a probable diagnosis), and if they were willing to participate. Diagnoses were made according to DSM-III-R criteria. In addition to the index probands, family members who fulfilled the criteria for a lifetime diagnosis of the respective category, schizophrenia (including schizoaffective disorder) or affective disorder, were included in the patient groups if they agreed to participate. Clinical assessment of inpatients was undertaken in the hospital where they were being treated. Outpatients and relatives were assessed in their homes or in the hospital, if they were willing to travel. Patients were excluded if they fulfilled the criteria for a comorbid diagnosis of substance dependence, neurological disorder, organic brain disorder, eye disorder, or any physical problem interfering with the execution of the eye tracking task.
By using this procedure, we recruited 54 patients with schizophrenia or schizoaffective disorder (Table 1). Among them, 33 (61%) were inpatients in the acute state of illness; the remaining individuals were outpatients. The mean age at illness onset was 25.1 years (SD=10.8), and they had suffered an average of 4.1 illness episodes (SD=3.7). The final group of 46 patients with affective disorders included 17 with major depression and 29 with bipolar disorder. Fourteen affective disorder patients (30%) were hospitalized for treatment at the time of testing, and 32 (70%) were currently in a remitted state but had a lifetime diagnosis of affective disorder. Their mean age at illness onset was 30.4 years (SD=10.1); before being tested they had gone through an average of 5.1 illness phases (SD=5.3).
Most patients were receiving psychotropic medication at the time of assessment. Of the patients with schizophrenia or schizoaffective disorder, 34 (63%) were receiving typical neuroleptics, 15 (28%) atypical neuroleptics, seven (13%) antidepressant medication, five (9%) lithium, six (11%) antiparkinsonian agents, and four (7%) benzodiazepines. Eight schizophrenia patients (15%) were free of any medication at the time of assessment. Of the affective disorder patients, 28 (61%) were receiving lithium, 15 (33%) antidepressants, 15 (33%) typical neuroleptics, one (2%) atypical neuroleptics, one (2%) antiparkinsonian agents, and one (2%) benzodiazepines. Nine (20%) were free of any medication. Three patients had a history of ECT but not during the 4 weeks before assessment.
Unaffected relatives of patients
After an initial family history evaluation of the patient, his or her family structure was established and provisional diagnoses were made for each relative. With the patient’s permission, relatives were then contacted. Relatives who lived within a radius of 200 km were personally contacted. If they were cooperative, they were visited by a study psychiatrist to make the clinical assessments. A subject was included in one of the groups of unaffected relatives if he or she was a first-degree biological relative of a patient included in the respective patient group, had no DSM-III-R axis I diagnosis and no schizotypal personality disorder at the time of assessment, and had no lifetime diagnosis of schizophrenia, schizoaffective disorder, or affective disorder. Additional exclusion criteria were the same as those for the patients. None of the unaffected relatives who were included took medication in the week before assessment. They were also strictly advised in advance not to drink any alcohol on the day of assessment and to drink only moderate amounts on the days before. By this recruitment strategy, a group of 43 unaffected first-degree relatives of patients with schizophrenia or schizoaffective disorder and a group of 36 unaffected first-degree relatives of affective disorder patients were obtained.
Healthy comparison subjects
The nonpsychiatric comparison group was recruited by word of mouth from the university hospital staff and from friends of the staff members. All family members of these candidates were carefully screened for a history of psychiatric diseases. In addition, the self-reported personal history of neurological disease, head trauma, and medication intake was assessed. Standardized interviews were conducted by a trained clinical psychologist (R.U.), either face-to-face or by telephone. In case a personal contact was not possible, family history information was obtained from a family member. The inclusion and exclusion criteria were the same as those for the unaffected relatives. The only difference was that the comparison group had no family history of schizophrenia or affective disorder. They were warned not to take medication, other drugs, or alcohol for 48 hours before the oculomotor tests. In the end, the comparison group comprised 84 healthy subjects.
Diagnostic Methods and Instruments
Each participant was interviewed face-to-face by a research psychiatrist using the German version of the Schedule for Affective Disorders and Schizophrenia—Lifetime Version Modified for the Study of Anxiety Disorders (29). In addition, the psychosis section of the Composite International Diagnostic Interview (30) was administered. In order to assess axis II disorders, the German version of the Structured Clinical Interview for DSM-III-R Personality Disorders (31) was used in the diagnostic procedure. Finally, symptoms were assessed by using the Operational Criteria Checklist (32). Best-estimate diagnoses (33) according to DSM-III-R criteria were made by the consensus of at least two experienced psychiatrists, one of whom was not directly involved in the present study, on the basis of all available information from clinical and standardized patient interviews, information obtained from relatives and/or friends, and review of medical charts.
Procedure for Eye Movement Testing
Eye movements were recorded by using portable equipment. This allowed testing of the subjects either in their homes or in the laboratory. In every case, a quiet room was used and illumination was kept dim. Visual stimuli were presented on the 10-inch screen of a laptop computer. The subject sat 42 cm in front of the screen with his or her head stabilized by a chin and forehead rest. The visual target was a bright circle subtending a visual angle of 0.6 degree on a dark background. The target moved horizontally across the screen transcribing a range of 22 degrees of visual arc. At the extremes, it paused for 0.5 second. During movement, the target velocity was constant (17.13 degrees/second). A trial consisted of 15 cycles (15 traverses in each direction), with a total duration of 54 seconds. Five trials were run with each subject. In the first and second trials, we used the standard task, which required the subject to follow the white circle with his or her eyes as closely as possible. In two additional runs, the eye tracking task was combined with auditory or visual distraction tasks (34). The subject was required to count target tones or target letters outside the white circle while doing the eye tracking task. In another trial, a visual monitoring task was used with letters superimposed on the target to be tracked. Before the test data were recorded, each subject received a training session. Horizontal eye movements were recorded by electro-oculography (EOG) using silver-silver chloride electrodes that were attached at the outer canthi of the eyes. The vertical EOG from electrodes above and below the right eye was used to identify eye blinks. A ground electrode was attached at the forehead. Electrode impedance was always kept below 5 kΩ. The low-pass filters were set at 100 Hz, and the time constant was 15 seconds. Analog signals were digitized online at a rate of 512 samples per second in each channel by using a 12-bit analog-to-digital converter board.
Analysis of Eye Movement Data
The horizontal EOG data were analyzed with semi-interactive software routinely used in our laboratory and described previously (34, 35). Briefly, the eye position signal was calibrated to ±11 degrees and corrected for phase error by means of cross-correlation. Then, 30 segments were cut from the whole horizontal EOG curve, 15 from the rightward and 15 from the leftward traverses. A segment began 400 msec after the onset of the target movement and ended 200 msec before the target stopped. This was done to exclude from analysis the acceleration and deceleration phases of the eye movement at the beginning and end of a target movement (36). After the first two segments were discarded from analysis, artifacts and saccades were removed from the remaining tracings. Rejection of artifacts (blinks, gross deviations of eye position by head and body movements, muscular artifacts, and nontracking phases) was done interactively by a person blind to the diagnosis of the subject (A.H.). Subsequently, the tracings were digitally low-pass filtered by using an upper frequency limit of 30 Hz (12). The velocity signal was computed as the first derivative of the filtered horizontal EOG position signal. Saccades were automatically identified by marking the intervals in the velocity signal that exceeded 40 degrees/second. Values adjacent to these high-velocity segments were also made invalid until the velocity signal was below 20 degrees/second. By using this procedure, saccades with amplitudes of 1 degree or larger could be reliably detected and removed. The remaining valid parts of the segments were regarded as true pursuit. The gain was then computed separately for each of the 28 segments as the mean velocity of valid intervals divided by the target velocity. Pursuit performance was determined as the mean gain in smooth pursuit eye movements across the 28 segments in each of the five tasks. A gain equal to 1.0 reflects perfect pursuit performance. Different types of saccades, such as catch-up saccades, anticipatory saccades, or square wave jerks, were not analyzed separately in this study because the focus was on the gain as the most pure measure of smooth pursuit.
Statistical Analysis
Overall pursuit performance was determined as the average gain from the five trials. We decided to collapse the data from the different conditions because performance was highly intercorrelated between conditions (Cronbach’s alpha=0.87 for the entire study group). The distributions of the resulting overall gains were nonnormal in three out of the five subject groups, as well as in the total group (Kolmogorov-Smirnov goodness of fit test, p<0.10). Skewness was greater than 1.0 in all raw data distributions. Therefore, the data were transformed before being subjected to further statistical analysis. As suggested by Box and Cox (37), the monotonic function f(x)=1/λ(xλ–1) was used. A lambda value was searched for that minimized skewness of the distributions. The criterion was a skewness less than 0.2 in each subgroup, as well as in the total group, and nonsignificant Kolmogorov-Smirnov z values (two-tailed asymptotic significance: p>0.20) in the subgroups and the total group. A lambda value of 9 fulfilled this criterion. After transformation of the raw data, an analysis of covariance (ANCOVA) was performed, with age as a covariate, to test group differences. The regression slopes of the five groups were similar and had overlapping confidence intervals. Planned contrasts were calculated to test specific hypotheses. Diagnostic specificity was considered to be supported if the gain in the smooth pursuit eye movements of the schizophrenia patients was lower than both that of the healthy comparison subjects and that of the affective disorder patients; familial specificity of gain deficits was considered to be supported if the unaffected first-degree relatives of the schizophrenia patients showed lower gains than both the healthy comparison subjects and the unaffected first-degree relatives of the affective disorder patients. An alpha level for significance of p<0.05 (two-tailed) was used.
Results
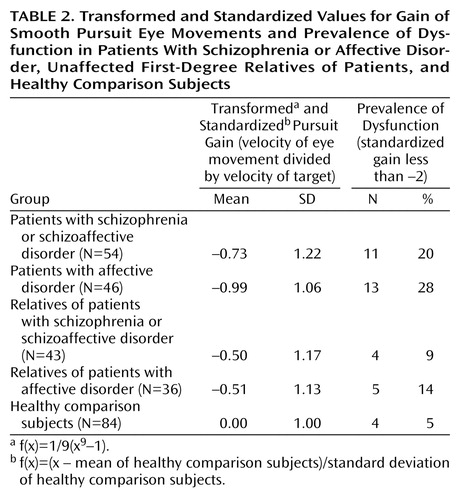
The groups differed in age and educational level but not in gender distribution and handedness (Table 1). The affective disorder patients and unaffected relatives of schizophrenia patients were older than the healthy comparison subjects. Since age showed a weak but significant correlation with eye tracking performance in the comparison group (r=–0.22, N=84, p=0.05), it was used as a covariate in tests of group differences in smooth pursuit eye movement gain. The patients with schizophrenia and their relatives were less educated than the healthy subjects. Education did not correlate with gain in the healthy subjects (r=0.006, N=84, p=0.95) and was not considered in further analyses. Figure 1 presents the distribution of the gains in each group. Table 2 shows the transformed values that were additionally normalized to the mean and the standard deviation of the healthy comparison group.
A one-way ANCOVA comparing the pursuit gains in the five diagnostic groups with age as a covariate showed highly significant group differences (F=6.0, df=4, 257, p<0.001). To test diagnostic specificity, the two patient groups and the healthy group were compared pairwise: the patients with schizophrenia or schizoaffective disorder had lower pursuit gain than the healthy subjects (F=14.5, df=1, 135, p<0.001) but did not differ from the affective disorder patients (F=0.1, df=1, 97, p=0.81). The affective disorder patients, however, had lower gain than the healthy group (F=19.5, df=1, 127, p<0.001). Familial specificity of pursuit gain deficits was tested in pairwise comparisons of the two groups of relatives and the healthy group: the unaffected relatives of the patients with schizophrenia or schizoaffective disorder had a lower gain than the healthy group (F=6.2, df=1, 124, p=0.02). No difference was found between the unaffected relatives of the patients with schizophrenia or schizoaffective disorder and the unaffected relatives of the affective disorder patients (F=0.3, df=1, 76, p=0.61). The relatives of the affective disorder patients, however, showed significantly worse pursuit performance than the comparison group (F=6.1, df=1, 117, p=0.02). Because data from biological relatives are not fully independent observations, a second ANCOVA was done with degrees of freedom computed on the basis of the number of families, instead of the number of individuals. Despite this very conservative approach, the pattern of results remained unchanged.
In order to test whether we would be justified in combining patients with bipolar disorder (N=29) and unipolar depression (N=17) into one group of affective disorder patients, these two subgroups were compared in additional analyses. They did not differ in age (t=–0.67, df=44, p=0.51) or in pursuit gain (t=–1.59, df=44, p=0.12). Further comparisons of the patients with schizoaffective disorder (N=16) and the combined group of those with all other subtypes of schizophrenia (N=38) were performed. Again, neither age (t=0.20, df=52, p=0.84) nor pursuit gain (t=0.77, df=52, p=0.44) showed a significant group difference.
Since dysfunction of smooth pursuit eye movement is frequently indicated in the literature as a categorical variable (present or absent), we also determined the prevalence rates across diagnostic groups. Dysfunction was defined by a z value less than –2 in relation to the normalized values (12). The prevalence estimates for the five groups are shown in Table 2.
Discussion
Familial specificity of deficits in gain of smooth pursuit eye movements, a putative vulnerability marker for schizophrenia, was tested by assessing unaffected biological relatives of patients with either schizophrenia spectrum disorders or affective disorders. The two groups of unaffected relatives showed comparable pursuit deficits. This lack of specificity of the pursuit dysfunction for schizophrenia is contradictory to the hypothesis commonly held in the literature. Whereas lower gain in relatives of patients with schizophrenia has already been reported (10, 13, 15), we know of no reports on gain measures in relatives of affective disorder patients. On the basis of the present results, we conclude that the pursuit gain indicates a dysfunction that aggregates in families of both schizophrenia and affective disorder patients.
Further evidence against diagnostic specificity of the pursuit deficit is the finding that patients with affective disorders differed from healthy subjects to an extent similar to that for schizophrenia patients. The prevalence rate was even slightly higher than in the schizophrenia group. In addition, the occurrence of dysfunction in smooth pursuit eye movement was not confined to either bipolar or unipolar disorder, as has been suggested in previous research (20, 38). The present data are in agreement with the results of Sweeney et al. (18, 23), who also used the gain measure as a specific indicator of the intactness of the pursuit function. The majority of previous studies that did not show pursuit deficits in affective disorder patients (12, 16, 17, 20) relied on global measures of the quality of smooth pursuit eye movements and did not directly assess pursuit function. Because the hypothesis that the pursuit deficit in affective disorder patients is more a state than a trait marker has been favored (19), inpatients were compared with patients either being treated in an outpatient setting or not being treated because their disorders were in remission at the time of testing. In fact, the acutely ill inpatients had lower gains than the outpatients and the untreated group (t=2.49, df=44, p=0.02). However, even the patients with nonacute illness showed a normalized gain of –0.78, which is almost identical to that of the schizophrenia group in our study. The failure to find diagnostic specificity, i.e., statistically better pursuit performance in affective disorder patients or their relatives than in patients with schizophrenia or schizoaffective disorder or their relatives, cannot be due to small study groups and subsequently insufficient power of statistics because the affective disorder patients and their relatives showed numerically even lower gains than the schizophrenia patients and their relatives, respectively.
The present study exclusively focused on gain as an indicator of the oculomotor pursuit system because this measure is clearly favored in the neuro-ophthalmic literature (27, 28). Among researchers in the psychiatric field, a debate has emerged about the sensitivity of different performance measures for the eye tracking task (39). In a direct comparison (22), gain was shown to be superior to other measures in discriminating schizophrenia patients and healthy subjects; however, another study (14) demonstrated that qualitative ratings were more sensitive than specific quantitative measures to the deficits of relatives of schizophrenia patients. It seems possible that global qualitative ratings contain more information than any one of the specific measures, but they have the serious disadvantage that they are unable to differentiate between inputs from different oculomotor or nonoculomotor sources. There is also some evidence that saccadic measures, such as the frequencies of catch-up and anticipatory saccades, might reflect deficits that are more specific to schizophrenia (18, 21, 38). A limitation of this study is that it did not evaluate a full set of specific oculomotor measures and, therefore, cannot contribute to these methodological issues. The present results strongly suggest that low pursuit gain is not specific to schizophrenia, whereas conclusions about primary abnormalities in saccadic mechanisms during pursuit cannot be drawn.
Schizophrenia is probably a heterogeneous disorder with uncertain phenotypic expressions. Genetic transmission is assumed to be complex, and this makes it difficult to identify the genes contributing to the disease. A polygenic model with several genes predisposing to schizophrenia is currently seen as most compatible with the data (40, 41). The search for endophenotypes (4) may help to identify these genes. Pursuit dysfunction is at present one of the best established endophenotypes, and a linkage study (42) has already identified a region on chromosome 6p for the underlying gene. It is interesting that there was no linkage between the genetic marker and the clinical diagnosis of schizophrenia. The modest sensitivity of the gain difference (reflecting the pursuit dysfunction) found in the present study and in earlier studies for the clinical diagnosis of schizophrenia allows us to conclude that the underlying gene(s) make(s) a nonnecessary contribution to the polygenic etiology. The diagnostic and familial nonspecificity, on the other hand, can be interpreted as an indication of shared genetic factors in schizophrenia and affective disorders. It seems possible that either the smooth pursuit eye movement dysfunction adds nonspecific disturbance to the more specific genetic and nongenetic causes of the psychotic disorders, thereby increasing the disease risk in the sense of a threshold model, or there are specific interactions of the putative gene for smooth pursuit eye movement disturbance with other causal genes resulting in the emergence of subtypes of schizophrenia and affective disorders. Evidence for an association of eye tracking disorder and deficit syndromes in schizophrenia patients has been reported (43). The view of a partly shared etiology of schizophrenias and affective disorder is also in accordance with the results of several epidemiologic family studies (44–46) that have shown that the relative risks for affective disorders are higher in family members of index probands with schizophrenia. The present finding of significant gain deficits in both schizophrenia and affective disorder patients, as well as in their unaffected biological relatives, provides further evidence for that view and constitutes a major challenge for the Kraepelinian dichotomy (47).
We believe this to be the first study of gain measures in biological relatives of affective disorder patients, and there is a need for replication of the present findings. Limitations of the study include the relatively low precision of the EOG measurements, the use of only one target velocity and one performance measure, and the possibility of uncontrolled effects of medications. Future family studies should record eye movements with high-resolution equipment, evaluate a comprehensive set of eye movement variables, and adhere to standardized procedures to increase comparability of results.
 |
 |
Presented in part at the Tenth European Conference on Eye Movements, Utrecht, the Netherlands, Sept. 23–25, 1999. Received Aug. 28, 2001; revision received July 25, 2002; accepted Oct. 15, 2002. From the Psychiatrische Klinik, Ludwig-Maximilians-Universität München. Address reprint requests to Dr. Kathmann, Department of Psychology, Humboldt University, Hausvogteiplatz 5-7, D-10117 Berlin, Germany; [email protected] (e-mail). Supported by the Deutsche Forschungsgemeinschaft (grant Bo 710/2). The authors thank Sabine Wienecke, M.D., Veronika Thoma, M.D., and Barbara Thaler, M.D., for interviewing the patients; Rolf Engel, Ph.D., for help with statistical analysis of the data; and Claudia Schöchlin, Ph.D., for help in programming the eye movement analysis software.
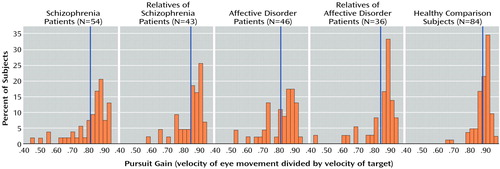
Figure 1. Distributions of Raw Values for Gain of Smooth Pursuit Eye Movements in Patients With Schizophrenia or Affective Disorder, Unaffected First-Degree Relatives of Patients, and Healthy Comparison Subjectsa
aGain values ranged between 0.40 and 1.00. The vertical blue lines denote group mean values.
1. Clementz BA, Sweeney JA: Is eye movement dysfunction a biological marker for schizophrenia? a methodological review. Psychol Bull 1990; 108:77-92Crossref, Medline, Google Scholar
2. Levy DL, Holzman PS, Matthysse S, Mendell NR: Eye tracking and schizophrenia: a selective review. Schizophr Bull 1994; 20:47-62Crossref, Medline, Google Scholar
3. Tsuang MT, Lyons MJ, Faraone SV: Clinical phenotypes: problems in diagnosis, in Genetic Research in Psychiatry. Edited by Mendlewicz J, Hippius H. Berlin, Springer, 1992, pp 173-187Google Scholar
4. Leboyer M, Bellivier F, Nosten-Bertrand M, Jouvent R, Pauls D, Mallet J: Psychiatric genetics: search for phenotypes. Trends Neurosci 1998; 21:102-105Crossref, Medline, Google Scholar
5. Iacono WG: Identifying psychophysiological risk for psychopathology: examples from substance abuse and schizophrenia research. Psychophysiology 1998; 35:621-637Crossref, Medline, Google Scholar
6. Holzman PS, Kringlen E, Levy DL, Haberman SJ: Deviant eye tracking in twins discordant for psychosis: a replication. Arch Gen Psychiatry 1980; 37:627-631Crossref, Medline, Google Scholar
7. Bell BB, Abel LA, Li W, Christian JC, Yee RD: Concordance of smooth pursuit and saccadic measures in normal monozygotic twin pairs. Biol Psychiatry 1994; 36:522-526Crossref, Medline, Google Scholar
8. Holzman PS, Proctor LR, Levy DL, Yasillo NJ, Meltzer HY, Hurt SW: Eye-tracking dysfunctions in schizophrenic patients and their relatives. Arch Gen Psychiatry 1974; 31:143-151Crossref, Medline, Google Scholar
9. Holzman PS, Solomon CM, Levin S, Waternaux CS: Pursuit eye movement dysfunctions in schizophrenia: family evidence for specificity. Arch Gen Psychiatry 1984; 41:136-139Crossref, Medline, Google Scholar
10. Clementz BA, Sweeney JA, Hirt M, Haas G: Pursuit gain and saccadic intrusions in first-degree relatives of probands with schizophrenia. J Abnorm Psychol 1990; 99:327-335Crossref, Medline, Google Scholar
11. Blackwood DH, St Clair DM, Muir WJ, Duffy JC: Auditory P300 and eye tracking dysfunction in schizophrenic pedigrees. Arch Gen Psychiatry 1991; 48:899-909Crossref, Medline, Google Scholar
12. Iacono WG, Moreau M, Beiser M, Fleming JA, Lin TY: Smooth-pursuit eye tracking in first-episode psychotic patients and their relatives. J Abnorm Psychol 1992; 101:104-116Crossref, Medline, Google Scholar
13. Arolt V, Lencer R, Nolte A, Pinnow M, Schwinger E: Eye tracking dysfunction in families with multiple cases of schizophrenia. Eur Arch Psychiatry Clin Neurosci 1996; 246:175-181Crossref, Medline, Google Scholar
14. Keefe RSE, Silverman JM, Mohs RC, Siever LJ, Harvey PD, Friedman L, Lees Roitman, SE, DuPre RL, Smith CJ, Schmeidler J, Davis KL: Eye tracking, attention, and schizotypal symptoms in nonpsychotic relatives of patients with schizophrenia. Arch Gen Psychiatry 1997; 54:169-176Crossref, Medline, Google Scholar
15. Lencer R, Malchow CP, Krecker K, Nolte A, Pinnow M, von Siefart SZ, Schwinger E, Arolt V: Smooth pursuit performance in families with multiple occurrence of schizophrenia and nonpsychotic families. Biol Psychiatry 1999; 45:694-703Crossref, Medline, Google Scholar
16. Amador XF, Sackeim HA, Mukherjee S, Halperin R, Neeley P, Maclin E, Schnur D: Specificity of smooth pursuit eye movement and visual fixation abnormalities in schizophrenia: comparison to mania and normal controls. Schizophr Res 1991; 5:135-144Crossref, Medline, Google Scholar
17. Holzman PS, O’Brian C, Waternaux C: Effects of lithium treatment on eye movements. Biol Psychiatry 1991; 29:1001-1015Crossref, Medline, Google Scholar
18. Sweeney JA, Clementz BA, Haas GL, Escobar MD, Drake K, Frances AJ: Eye tracking dysfunction in schizophrenia: characterization of component eye movement abnormalities, diagnostic specificity, and the role of attention. J Abnorm Psychol 1994; 103:222-230Crossref, Medline, Google Scholar
19. Malaspina D, Amador XF, Coleman EA, Mayr TL, Friedman JH, Sackeim HA: Smooth pursuit eye movement abnormality in severe major depression: effects of ECT and clinical recovery. J Neuropsychiatry Clin Neurosci 1994; 6:36-42Crossref, Medline, Google Scholar
20. Iacono WG, Peloquin LJ, Lumry AE, Valentine RH, Tuason VB: Eye tracking in patients with unipolar and bipolar affective disorders in remission. J Abnorm Psychol 1982; 91:35-44Crossref, Medline, Google Scholar
21. Abel LA, Friedman L, Jesberger J, Malki A, Meltzer HY: Quantitative assessment of smooth pursuit gain and catch-up saccades in schizophrenia and affective disorders. Biol Psychiatry 1991; 29:1063-1072Crossref, Medline, Google Scholar
22. Friedman L, Jesberger JA, Siever LJ, Thompson P, Mohs R, Meltzer HY: Smooth pursuit performance in patients with affective disorders or schizophrenia and normal controls: analysis with specific oculomotor measures, RMS error and qualitative ratings. Psychol Med 1995; 25:387-403Crossref, Medline, Google Scholar
23. Sweeney JA, Luna B, Haas GL, Keshavan MS, Mann JJ, Thase ME: Pursuit tracking impairments in schizophrenia and mood disorders: step-ramp studies with unmedicated patients. Biol Psychiatry 1999; 46:671-680Crossref, Medline, Google Scholar
24. Levy DL, Dorus E, Shaughnessy R, Yasillo NJ, Pandey GN, Janicak PG, Gibbons RD, Gaviria M, Davis JM: Pharmacologic evidence for specificity of pursuit dysfunction to schizophrenia: lithium carbonate associated with abnormal pursuit. Arch Gen Psychiatry 1985; 42:335-341Crossref, Medline, Google Scholar
25. Gooding DC, Iacono WG, Katsanis J, Beiser M, Grove WM: The association between lithium carbonate and smooth pursuit eye tracking among first-episode patients with psychotic affective disorders. Psychophysiology 1993; 30:3-9Crossref, Medline, Google Scholar
26. Levy DL, Yasillo NJ, Dorus E, Shaughnessy R, Gibbons RD, Peterson J, Janicak PG, Gaviria M, Davis JM: Relatives of unipolar and bipolar patients have normal pursuit. Psychiatry Res 1983; 10:285-293Crossref, Medline, Google Scholar
27. Abel LA, Ziegler AS: Smooth pursuit eye movements in schizophrenics—what constitutes quantitative assessment? Biol Psychiatry 1988; 24:747-761Crossref, Medline, Google Scholar
28. Leigh RJ, Zee DS: The Neurology of Eye Movements, 2nd ed. Philadelphia, FA Davis, 1991Google Scholar
29. Mannuzza S, Fyer AJ, Klein DF, Endicott J: The Schedule for Affective Disorders and Schizophrenia—Lifetime Version Modified for the Study of Anxiety Disorders (SADS-LA): rationale and conceptual development. J Psychiatr Res 1986; 20:317-325Crossref, Medline, Google Scholar
30. World Health Organization: Composite International Diagnostic Interview (CIDI), version 1.0. Geneva, WHO, 1990Google Scholar
31. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II). Washington, DC, American Psychiatric Press, 1990Google Scholar
32. McGuffin P, Farmer A, Harvey I: A polydiagnostic application of operational criteria in psychotic illness: development and reliability of the OPCRIT system. Arch Gen Psychiatry 1991; 48:764-770Crossref, Medline, Google Scholar
33. Leckman JF, Sholomskas D, Thompson WD, Belanger A, Weissman MM: Best estimate of lifetime psychiatric diagnoses: a methodological study. Arch Gen Psychiatry 1982; 39:879-883Crossref, Medline, Google Scholar
34. Kathmann N, Hochrein A, Uwer R: Effects of dual task demands on the accuracy of smooth pursuit eye movements. Psychophysiology 1999; 36:158-163Crossref, Medline, Google Scholar
35. Kathmann N, Wagner M, Rendtorff N, Schöchlin C, Engel RR: Information processing during eye tracking as revealed by event-related potentials in schizophrenics, alcoholics, and healthy controls. Schizophr Res 1995; 16:145-156Crossref, Medline, Google Scholar
36. Robinson DA, Gordon JL, Gordon SE: A model of the smooth pursuit eye movement system. Biol Cybern 1986; 55:43-57Crossref, Medline, Google Scholar
37. Box GEP, Cox DR: An analysis of transformations. J R Stat Soc 1964; 26:211-243Google Scholar
38. Rosenberg DR, Sweeney JA, Squires-Wheeler E, Keshavan MS, Cornblatt BA, Erlenmeyer-Kimling L: Eye-tracking dysfunction in offspring from the New York High-Risk Project: diagnostic specificity and the role of attention. Psychiatry Res 1997; 66:121-130Crossref, Medline, Google Scholar
39. Ross DE, Thaker GK, Buchanan RW, Lahti AC, Conley R, Medoff D: Specific measures account for most of the variance in qualitative ratings of smooth pursuit eye movements in schizophrenia. Arch Gen Psychiatry 1998; 55:184-185Crossref, Medline, Google Scholar
40. McGue M, Gottesman II, Rao DC: Resolving genetic models for the transmission of schizophrenia. Genet Epidemiol 1985; 2:99-110Crossref, Medline, Google Scholar
41. Portin P, Alanen YO: A critical review of genetic studies of schizophrenia, II: molecular genetic studies. Acta Psychiatr Scand 1997; 95:73-80Crossref, Medline, Google Scholar
42. Arolt V, Lencer R, Nolte A, Muller-Myhsok B, Purmann S, Schurmann M, Leutelt J, Pinnow M, Schwinger E: Eye tracking dysfunction is a putative phenotypic susceptibility marker of schizophrenia and maps to a locus on chromosome 6p in families with multiple occurrence of the disease. Am J Med Genet 1996; 67:564-579Crossref, Medline, Google Scholar
43. Ross DE, Thaker GK, Buchanan RW, Kirkpatrick B, Lahti AC, Medoff D, Bartko JJ, Goodman J, Tien A: Eye tracking disorder in schizophrenia is characterized by specific ocular motor defects and is associated with the deficit syndrome. Biol Psychiatry 1997; 42:781-796Crossref, Medline, Google Scholar
44. Gershon ES, de Lisi LE, Hamovit J, Nurnberger JI, Maxwell ME, Schreiber J, Dauphinais D, Dingman CW, Guroff JJ: A controlled family study of chronic psychosis. Arch Gen Psychiatry 1988; 45:328-336Crossref, Medline, Google Scholar
45. Maier W, Lichtermann D, Minges J, Hallmayer J, Heun R, Benkert O, Levinson DF: Continuity and discontinuity of affective disorders and schizophrenia: results of a controlled family study. Arch Gen Psychiatry 1993; 50:871-883Crossref, Medline, Google Scholar
46. Kendler KS, Gardner CO: The risk for psychiatric disorders in relatives of schizophrenic and control probands: a comparison of three independent studies. Psychol Med 1997; 27:411-419Crossref, Medline, Google Scholar
47. Kraepelin E: Psychiatrie—Ein Lehrbuch für Studierende und Ärzte. Leipzig, Barth, 1899Google Scholar



