Parahippocampal Gray Matter Density in Panic Disorder: A Voxel-Based Morphometric Study
Abstract
OBJECTIVE: The authors examined possible cerebral gray matter abnormalities in patients with panic disorder. METHOD: Gray matter concentration in 18 panic disorder outpatients and 18 healthy subjects was compared by using a voxel-based morphometry approach. RESULTS: Gray matter density of the left parahippocampal gyrus was significantly lower in patients with panic disorder compared with healthy subjects. CONCLUSIONS: This result provides further support for the involvement of the parahippocampal area in the pathophysiology of panic disorder.
Qualitative magnetic resonance imaging (MRI) studies in panic disorder have shown a variety of brain abnormalities, mostly involving the temporal lobes and mainly located in the mesiotemporal area (1, 2). In addition, a high frequency of septohippocampal abnormalities has been reported in association with nonepileptic EEG abnormalities in patients with panic disorder (3). The only study that used a more reliable approach, quantitative MRI (4), found a bilateral decrease in temporal lobe volume in panic disorder patients.
To date, voxel-based morphometry has been applied in the study of psychiatric and neurologic conditions, given its power for detecting changes in gray or white matter density of the brain (5). In this article, we report the power of voxel-based morphometry to identify structural cerebral abnormalities in patients with panic disorder.
Method
Eighteen right-handed outpatients (11 women and seven men; mean age=36.8 years, SD=11.3) were recruited for the study after having been diagnosed with panic disorder according to the Structured Clinical Interview for DSM-IV (SCID) (6). Fifteen out of 18 had some degree of agoraphobia. None of the patients had a past or current diagnosis of other axis I disorders (excluding some mild cases of specific phobia), traumatic brain injury, or other neurological diseases.
Eighteen right-handed healthy subjects (10 women and eight men) of a similar age (mean=36.7, SD=8.8) and educational level were recruited among hospital staff after undergoing a psychiatric interview and the SCID to rule out current or past medical and psychiatric diagnoses.
This study was approved by the Local Research Ethics Committee of our university hospital. All subjects signed written informed consent agreements following detailed explanation of the study and procedure.
Axial three-dimensional T1-weighted spoiled gradient echo MRI scans were performed on a GE Signa 1.5-T scanner (TR/TE=12.5/2.2 msec, flip angle=20°, field of view=24 cm, slice thickness=contiguous 1.2 mm, number of excitations=3, matrix=256×160, voxel resolution=1.2×0.9×0.9 mm3).
Voxel-based morphometry is a method for voxel-wise between-group comparison of local gray matter concentrations (5). The three-dimensional MRI data sets were analyzed by using SPM 99 software (Statistical Parametric Mapping, Wellcome Department of Cognitive Neurology, University College London), as previously described (7).
The T1-weighted images were transformed into standard Montreal Neurological Institute space by using an automated spatial normalization (8). The normalized whole-brain images were automatically segmented into an image representing probability maps for gray matter and finally smoothed by using a 12-mm full-width half-maximum isotropic Gaussian kernel.
Using the SPM 99 compare-populations analysis, we performed two one-sided t tests (healthy subjects > patients and patients > healthy subjects) in the general gray matter study, following a procedure described elsewhere (7). We derived p values for both differences in gray matter density on a voxel-by-voxel basis and also for the spatial extent of clusters of affected voxel. Only those clusters exceeding a size of 10 voxels were analyzed.
Results
Voxel-based morphometry analysis revealed gray matter deficits in the left parahippocampal gyrus of the patients (Figure 1). No other brain regions showed significant differences (for either healthy subjects > patients or patients > healthy subjects) when the data were corrected for multiple comparisons. Gray matter differences could also be seen in other structures when uncorrected p values (p<0.001) were used. Regions in which gray matter concentrations were greater in the healthy subjects than in the patients were the left cuneus, right middle temporal gyrus, right inferior temporal gyrus, hypothalamus, right parahippocampal gyrus, right thalamus, and left and right cerebellum. Regions in which gray matter concentrations were greater in the patients than in the healthy subjects were the left middle temporal gyrus and left angular gyrus.
Discussion
We found left parahippocampal gyrus gray matter deficits in our group of panic disorder patients. To our knowledge, this is the first study to analyze patients with panic disorder by using voxel-based morphometry.
Our results are relevant in view of previous neuroimaging findings. On the one hand, panic disorder has been associated with a variety of structural brain abnormalities, mostly involving the temporal lobes (1–4). On the other hand, functional neuroimaging studies (PET and SPECT) have provided strong evidence for an abnormal function of the temporal lobe. Reiman et al. (9) found an abnormal hemispheric asymmetry of parahippocampal blood flow and oxygen metabolism in panic disorder patients in the resting, nonpanic states. Despite some relevant methodological limitations, such asymmetry was interpreted as an abnormal increase in right parahippocampal measurements. Glucose metabolism asymmetry in both the hippocampal and parahippocampal structures was later reported (10), which also suggests an increase in glucose metabolic rates on the right side. Similar results were found studying asymptomatic, imipramine-treated panic disorder patients (11), suggesting that such abnormality could reflect a trait marker for the illness. However, the picture is not that clear, since lower perfusion indices both in the right and left hippocampal regions (12) and a significant increase in glucose metabolism in the left hippocampus and parahippocampal area in women (13) have been also reported in panic disorder. Despite these rather inconsistent findings, our results could suggest that the left-to-right parahippocampal asymmetries described in most functional neuroimaging studies reflect compensatory mechanisms possibly due to gray matter deficits in the left parahippocampal region.
In summary, our results support the involvement of the parahippocampal gyrus in the pathophysiology of panic disorder. However, further studies with larger patient group sizes seem mandatory to clarify possible clinical differences (in terms of panic disorder, panic disorder with agoraphobia, and agoraphobia without panic disorder) and gender differences and to elucidate whether parahippocampal gray matter deficits precede the onset of the disorder or appear as a consequence of it.
Received April 3, 2002; revisions received June 26 and July 31, 2002; accepted Aug. 17, 2002. From the Institut Clínic de Psiquiatria i Psicologia, Corporació Sanitària Clínic; the Centre de Diagnòstic per la Imatge, Corporació Sanitària Clínic, I.D.I.B.A.P.S., Barcelona, Catalonia (Spain); and the Departament de Psiquiatria i Psicobiologia Clínica, Universitat de Barcelona, Barcelona, Catalonia (Spain). Address reprint requests to Dr. Massana, Institut Clínic de Psiquiatria i Psicologia, Corporació Sanitària Clínic, Villarroel 170, 08036 Barcelona, Catalonia, Spain; [email protected] (e-mail). Supported by research grants (99/0191 and 01/1503) from the Fondo de Investigaciones Sanitarias, Instituto de Salud Carlos III, Madrid.
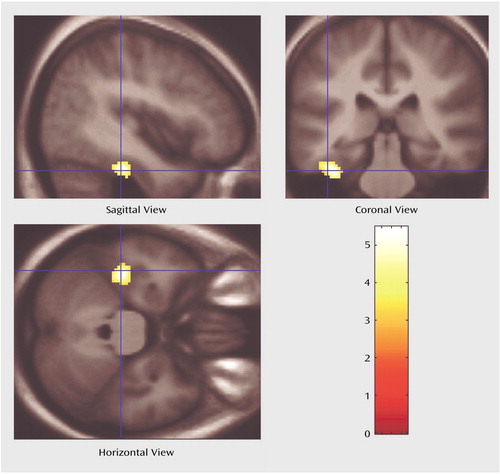
Figure 1. Voxel-Based Morphometric Analysis of Regional Gray Matter Differences Between Patients With Panic Disorder (N=18) and Healthy Comparison Subjects (N=18)a
aImages depict a 230-voxel cluster in the left parahippocampal gyrus (Talairach coordinates [x, y, z]: –36, –24, –22) in which gray matter density in the panic disorder patients relative to the comparison subjects was significantly lower after correction for multiple comparisons (t=5.47, df=34, p<0.05).
1.. Ontiveros A, Fontaine R, Breton G, Elie R, Fontaine S, Dery R: Correlation of severity of panic disorder and neuroanatomical changes on magnetic resonance imaging. J Neuropsychiatry Clin Neurosci 1989; 1:404-408Crossref, Medline, Google Scholar
2.. Fontaine R, Breton G, Dery R, Fontaine S, Elie R: Temporal lobe abnormalities in panic disorder: an MRI study. Biol Psychiatry 1990; 27:304-310Crossref, Medline, Google Scholar
3.. Dantendorfer K, Prayer D, Kramer J, Amering M, Baischer W, Berger P, Schoder M, Steinberger K, Windhaber J, Imhof H, Katschnig H: High frequency of EEG and MRI brain abnormalities in panic disorder. Psychiatry Res 1996; 68:41-53Crossref, Medline, Google Scholar
4.. Vythilingam M, Anderson ER, Goddard A, Woods SW, Staib LH, Charney DS, Bremner JD: Temporal lobe volume in panic disorder—a quantitative magnetic resonance imaging study. Psychiatry Res 2000; 99:72-82Crossref, Google Scholar
5.. Ashburner J, Friston KJ: Voxel-based morphometry—the methods. Neuroimage 2000; 11:805-821Crossref, Medline, Google Scholar
6.. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-IV (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
7.. Wilke M, Kaufmann C, Grabner A, Pütz B, Wetter TC, Auer DP: Gray matter-changes and correlates of disease severity in schizophrenia: a statistical parametric mapping study. Neuroimage 2001, 13:814-824Google Scholar
8.. Ashburner J, Friston KJ: Nonlinear spatial normalization using basis functions. Hum Brain Mapp 1999; 7:254-266Crossref, Medline, Google Scholar
9.. Reiman EM, Raichle ME, Robins E, Butler FK, Herscovitch P, Fox P, Perlmutter J: The application of positron emission tomography to the study of panic disorder. Am J Psychiatry 1986; 143:469-477Link, Google Scholar
10.. Nordahl TE, Semple WE, Gross M, Mellman TA, Stein MB, Goyer P, King AC, Uhde TW, Cohen RM: Cerebral glucose metabolic differences in patients with panic disorder. Neuropsychopharmacology 1990; 3:261-272Medline, Google Scholar
11.. Nordahl TE, Stein MB, Benkelfat C, Semple WE, Andreason P, Zametkin A, Uhde TW, Cohen RM: Regional cerebral metabolic asymmetries replicated in an independent group of patients with panic disorder. Biol Psychiatry 1998; 44:998-1006Crossref, Medline, Google Scholar
12.. De Cristofaro MTR, Sessarego A, Pupi A, Biondi F, Faravelli C: Brain perfusion abnormalities in drug-naive lactate-sensitive panic patients: a SPECT study. Biol Psychiatry 1993; 33:505-512Crossref, Medline, Google Scholar
13.. Bisaga A, Katz JL, Antonini A, Wright CE, Margouleff C, Gorman JM, Eidelberg D: Cerebral glucose metabolism in women with panic disorder. Am J Psychiatry 1998; 155:1178-1183Link, Google Scholar



