Differentiation of Geriatric Major Depression From Alzheimer’s Disease With CSF Tau Protein Phosphorylated at Threonine 231
Abstract
OBJECTIVE: Differentiation of geriatric major depression from Alzheimer’s disease is hampered by overlapping symptoms. Increased CSF concentrations of tau protein phosphorylated at threonine 231 (p-tau231) have been suggested as a biomarker for Alzheimer’s disease. The authors asked whether p-tau231 levels improve the differential diagnosis between geriatric major depression and Alzheimer’s disease. METHOD: Included were 34 depression subjects, 64 with probable Alzheimer’s disease, 17 with possible Alzheimer’s disease, and 21 healthy comparison subjects. P-tau231 concentrations were measured with an enzyme-linked immunosorbent assay. RESULTS: P-tau231 levels were significantly higher in Alzheimer’s disease than in geriatric major depression patients and healthy comparison subjects. For differentiation of probable Alzheimer’s disease from major depression, p-tau231 correctly allocated 87% of subjects. When possible mild Alzheimer’s disease was compared to major depression, p-tau231 correctly allocated 78% of subjects. CONCLUSIONS: CSF p-tau231 should be evaluated as a potential biological marker for differentiation of geriatric depression from Alzheimer’s disease.
One of the most challenging diagnostic issues in psychiatry is to differentiate major depression from mild to moderate Alzheimer’s disease in the elderly. Clinical symptoms overlap considerably, and often only follow-up allows clinical differentiation between both entities. In major depression, deficits in various cognitive domains may be present (1). On the other hand, key symptoms of major depression occur in patients with mild to moderate Alzheimer’s disease, such as depressed mood, apathy, social restraint, or loss of interests. Apathy, for example, has been detectable in 37% of Alzheimer’s disease patients as well as in 32% of depressed nondemented patients (2). Correct and early diagnosis of either major depression or Alzheimer’s disease is crucial with respect to prognosis and specific therapy. Until now, no accurate biological marker has been established to support the differential diagnosis of major depression and Alzheimer’s disease.
Recently, tau protein phosphorylated at threonine 231 (p-tau231) in CSF has been studied as a putative marker of Alzheimer’s disease. Measurement of p-tau231 levels has demonstrated high sensitivity and specificity in distinguishing Alzheimer’s disease patients from healthy comparison subjects and subjects with other neurological disorders and other dementias, especially frontotemporal dementia (3). Moreover, CSF p-tau231 concentrations were elevated in subjects at risk of Alzheimer’s disease and correlated with progressive cognitive decline in this group (4). CSF p-tau231 concentrations have declined intra-individually during the clinical progression of Alzheimer’s disease (5). The speed of the decline of p-tau231 levels has been inversely correlated with scores on the Mini-Mental State Examination (MMSE) at baseline. P-tau231 levels, however, remained high throughout the course of Alzheimer’s disease compared to those in healthy comparison subjects. These studies indicate that p-tau231 levels may be a useful biomarker for Alzheimer’s disease, particularly in the early clinical stages of Alzheimer’s disease.
We hypothesized that p-tau231 levels could accurately discriminate between major depression and Alzheimer’s disease because they detect an early and specific feature of the pathophysiology of Alzheimer’s disease (6). In this pilot study, we investigated CSF p-tau231 levels in geriatric major depression, as well as in mild to moderate Alzheimer’s disease and in very mild Alzheimer’s disease, in an independent study group.
Method
Thirty-four inpatients with major depression were diagnosed according to DSM-IV criteria (age: mean=65.4 years, SD=12.1; 24 women). A total of 64 subjects were diagnosed as having probable Alzheimer’s disease (age: mean=68.8 years, SD=9.7; 36 women) and 17 as having very mild impairment with possible Alzheimer’s disease (age: mean=71.9 years, SD=8.1; 11 women), according to criteria of the National Institute of Neurological and Communicative Disorders and Stroke (7). There were 21 healthy subjects in the study (age: mean=57.7 years, SD=14.2; eight women). On the MMSE (8), the subjects with major depression scored a mean of 27.8 points (SD=2.3), and the subjects with probable Alzheimer’s disease scored a mean of 20.1 points (SD=3.8) (>12 points). The subjects with possible Alzheimer’s disease scored a mean of 27.7 points (SD=1.2), and the healthy comparison subjects scored a mean of 29.0 points (SD=0.8). A total of 92.8% of the subjects with major depression or possible Alzheimer’s disease were in the same MMSE score range of 26 to 30 points.
The participants did not have any current unstable medical conditions. A total of 36% of the subjects with major depression, 14% of the healthy comparison subjects, 36% of the subjects with probable Alzheimer’s disease, and 50% of the subjects with possible Alzheimer’s disease were suffering from hypertension. A total of 8% of the subjects with major depression, 19% of the healthy comparison subjects, 5% of the subjects with probable Alzheimer’s disease, and 10% of the subjects with possible Alzheimer’s disease had diabetes mellitus. A total of 56% of the subjects with major depression, 24% of the healthy comparison subjects, 40% of the subjects with probable Alzheimer’s disease, and 70% of the subjects with possible Alzheimer’s disease were receiving treatment for somatic comorbidity. All of the major depression subjects, none of the healthy comparison subjects, 19% of subjects with probable Alzheimer’s disease, and 50% of the subjects with possible Alzheimer’s disease were receiving antidepressants.
The study protocol was approved by the local ethics committees and the institutional review boards of the participating centers. After a complete description of the study to the subjects, written informed consent was obtained.
CSF sampling and processing was performed as described in detail previously (3). P-tau231 levels were measured by using an enzyme-linked immunosorbent assay (ELISA, Molecular Geriatrics Corporation, Vernon Hills, Ill. [3]). Data are expressed as means and standard deviations.
Visual inspection of scatterplots and Schapiro-Wilks tests indicated that p-tau231 levels were not normally distributed between groups. Therefore, nonparametric Kruskal-Wallis analysis was used to test for differences in CSF p-tau231 levels, age, and MMSE scores over all groups, followed by pairwise comparisons between groups with the Mann-Whitney U test. To test for gender differences, chi-square tests were used. Correlations between p-tau231 concentrations and group characteristics were assessed with Spearman’s rank correlations. To determine the discriminative power of p-tau231 levels between Alzheimer’s disease and major depression subjects, sensitivity and specificity levels and numbers of correctly allocated cases were calculated by using receiver-operating-characteristic-curve analysis. The receiver-operating-characteristic curve is the plot of sensitivity versus the false positive rate (1–specificity) (9). Receiver-operating-characteristic curves can be used to determine cutoff values for a given sensitivity or specificity level. They were further used to determine the cutoff that maximizes the sum of sensitivity and specificity. The significance level was set at p<0.05.
Results
Major depression and probable and possible Alzheimer’s disease subjects did not differ in age or gender. Healthy comparison subjects were younger than the other groups (healthy comparison subjects versus major depression subjects: Mann-Whitney U=227, df=1, p<0.05; healthy comparison subjects versus probable Alzheimer’s disease subjects: Mann-Whitney U=318, df=1, p<0.001; healthy comparison subjects versus possible Alzheimer’s disease subjects (Mann-Whitney U=66, df=1, p=0.001).
We found a significant difference in p-tau231 levels (χ2=78, df=3, p<0.001) among all groups. P-tau231 levels (Figure 1) were significantly higher in probable Alzheimer’s disease subjects (mean=58 μl CSF equivalents, SD=29) than in major depression subjects (mean=10 μl CSF equivalents, SD=18) (Mann-Whitney U=147, df=1, p<0.001), healthy comparison subjects (mean=2 μl CSF equivalents, SD=9) (Mann-Whitney U=20, df=1, p<0.001), or possible Alzheimer’s disease subjects (mean=28 μl CSF equivalents, SD=30) (Mann-Whitney U=242, df=1, p<0.001). In patients with possible Alzheimer’s disease, p-tau231 levels were significantly higher than in major depression patients (Mann-Whitney U=158, df=1, p<0.01) and healthy comparison subjects (Mann-Whitney U=60, df=1, p<0.001). P-tau231 levels were higher in major depression patients than in healthy comparison subjects (Mann-Whitney U=226, df=1, p<0.05). Analyzes were repeated in subgroups matched for age and gender distribution. Differences between probable and possible Alzheimer’s disease patients and healthy comparison subjects remained unchanged (probable Alzheimer’s disease versus healthy comparison: Mann-Whitney U=5, df=1, p<0.001; possible Alzheimer’s disease versus healthy comparison: Mann-Whitney U=24, df=1, p<0.001). Differences in p-tau231 levels did not reach significance between major depression and healthy comparison subjects (Mann-Whitney U=176, df=1, p=0.27).
In discriminating probable Alzheimer’s disease from major depression subjects, at a cutoff of 18.1 μl CSF equivalents, p-tau231 level yielded a specificity of 85% and a sensitivity of 92%; 87% of the cases were correctly allocated. When possible Alzheimer’s disease patients were compared to major depression patients, at a cutoff of 7.6 μl CSF equivalents, the specificity for p-tau231 level was 71% at a sensitivity level of 82%. A total of 78% of the cases were correctly allocated. Correlations between p-tau231 level and age were found in major depression patients (rs=0.54, N=34, p<0.001) but not in Alzheimer’s disease patients and healthy comparison subjects. P-tau231 level was not correlated to MMSE score in either group.
Discussion
P-tau231 levels distinguished mild to moderate Alzheimer’s disease from major depression with a specificity level of 85%, a sensitivity level of 92%, and a correct classification rate of 87%. When major depression was compared to very mild Alzheimer’s disease, 78% of the cases were correctly allocated. To our knowledge, this is the first report on the use of CSF phosphorylated tau protein for the differential diagnosis of Alzheimer’s disease and geriatric major depression. The high level of discrimination of p-tau231 levels results because the threonine 231 epitope has been specifically implicated in the tau pathology of Alzheimer’s disease (6).
To further follow the diagnostic potential of p-tau231 levels in early and subtle clinical manifestations, we included a group of very mildly impaired Alzheimer’s disease patients whose MMSE scores were comparable to those of the major depression patients. P-tau231 levels still correctly allocated 78% of the cases. This result indicates that p-tau231 levels also have the potential to differentiate Alzheimer’s disease from major depression if MMSE scores do not.
Only subjects with a diagnosis of either major depression or Alzheimer’s disease were included in the present pilot study. Elevated levels of p-tau231 do not rule out major depression as a comorbidity in Alzheimer’s disease patients. When psychopathological symptoms overlap, however, high p-tau231 levels probably point to an underlying Alzheimer’s-disease-specific pathological process. None of our major depression patients was diagnosed as having possible or probable Alzheimer’s disease. The patients with major depression, however, tended to present higher p-tau231 levels than the healthy comparison subjects. Moreover, p-tau231 levels increased with age in the major depression group. Old age (10) and depression (11) have been reported to be risk factors for Alzheimer’s disease. Thus, underlying Alzheimer’s disease pathology and neurodegeneration at presymptomatic stages might have been present in some major depression patients. In future studies, major depression patients need to be clinically followed to investigate whether subjects with elevated p-tau231 levels might be at a higher risk for developing Alzheimer’s disease over time.
We suggest that p-tau231 concentrations should be further investigated as a tool to support the differential diagnosis of geriatric major depression and Alzheimer’s disease.
Received Sept. 27, 2001; revision received July 2, 2002; accepted July 8, 2002. From the Dementia Research Section and Memory Clinic, Alzheimer Memorial Center and Geriatric Psychiatry Branch, Department of Psychiatry, Ludwig-Maximilian University; the Molecular Geriatrics Corporation, Vernon Hills, Ill.; the Department of Geriatric Medicine, Tohoku University School of Medicine, Sendai, Japan; the Department of Neuroscience and Neurology, University Hospital, University of Kuopio, Kuopio, Finland; and the Brain Physiology and Metabolism Section, National Institute on Aging, NIH, Bethesda, Md. Address reprint requests to Dr. Buerger, Dementia Research Section and Memory Clinic, Alzheimer Memorial Center and Geriatric Psychiatry Branch, Department of Psychiatry, Ludwig-Maximilian University, Nussbaumstrasse 7, 80336 Munich, Germany; katharina. [email protected] (e-mail).Supported by grants from the Volkswagen Foundation (Dr. Hampel), Hirnliga (Drs. Hampel and Buerger), and the Foerderprogramm fuer Forschung und Lehre, Faculty of Medicine, Ludwig-Maximilian University (Drs. Buerger, Teipel, and Hampel).The authors thank F. Jancu, B. Riemenschneider, J. Wagner, and O. Pogarell, M.D., for clinical support, T. Nolde, H. Gluba, and H. Ott for technical assistance, and A.L.W. Bokde, Ph.D., for review of the article.
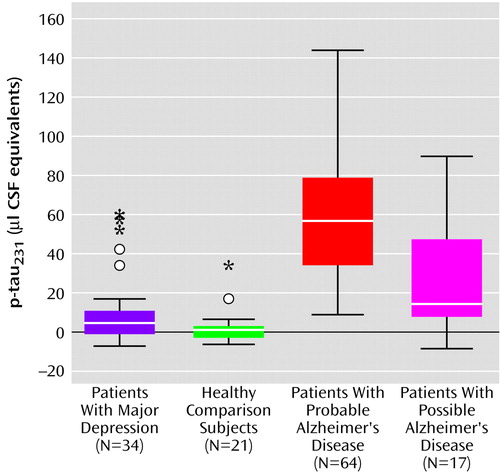
Figure 1. CSF Concentrations of Tau Protein Phosphorylated at Threonine 231 (p-tau231) in Patients With Major Depression, Healthy Comparison Subjects, and Patients With Probable or Possible Alzheimer’s Diseasea
aBoxes represent the median and the 25th and 75th percentiles; bars indicate the range of data distribution. Circles represent values more than 1.5 box heights away from the 25th or 75th percentile (outliers). Asterisks represent values more than 3 box heights away from the 25th or 75th percentile (extremes).
1. Christensen H, Griffiths K, Mackinnon A, Jacomb P: A quantitative review of cognitive deficits in depression and Alzheimer-type dementia. J Int Neuropsychol Soc 1997; 3:631-651Crossref, Medline, Google Scholar
2. Starkstein SE, Petracca G, Chemerinski E, Kremer J: Syndromic validity of apathy in Alzheimer’s disease. Am J Psychiatry 2001; 158:872-877Link, Google Scholar
3. Buerger K, Zinkowski R, Teipel SJ, Tapiola T, Arai H, Blennow K, Andreasen N, Hofmann-Kiefer K, DeBernardis J, Kerkman D, McCulloch C, Kohnken R, Padberg F, Pirttilä T, Schapiro M, Rapoport SI, Möller HJ, Davies P, Hampel H: Differential diagnosis of Alzheimer disease with cerebrospinal fluid levels of tau protein phosphorylated at threonine 231. Arch Neurol 2002; 59:1267-1272Crossref, Medline, Google Scholar
4. Buerger K, Teipel SJ, Zinkowski R, Blennow K, Arai H, Engel R, Hofmann-Kiefer K, McCulloch C, Ptok U, Heun R, Andreasen N, DeBernardis J, Kerkman D, Möller HJ, Davies P, Hampel H: CSF tau protein phosphorylated at threonine 231 correlates with cognitive decline in MCI subjects. Neurology 2002; 59:627-629Crossref, Medline, Google Scholar
5. Hampel H, Buerger K, Kohnken R, Teipel SJ, Zinkowski R, Rapoport SI, Möller HJ, Davies P: Tracking of Alzheimer’s disease with cerebrospinal fluid tau protein phosphorylated at threonine 231. Ann Neurol 2001; 49:545-546Crossref, Medline, Google Scholar
6. Vincent I, Zheng JH, Dickson DW, Kress Y, Davies P: Mitotic phosphoepitopes precede paired helical filaments in Alzheimer’s disease. Neurobiol Aging 1998; 19:287-296Crossref, Medline, Google Scholar
7. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM: Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34:939-944Crossref, Medline, Google Scholar
8. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189-198Crossref, Medline, Google Scholar
9. Metz CE: Basic principles of ROC analysis. Semin Nucl Med 1978; 8:283-298Crossref, Medline, Google Scholar
10. Jorm AF, Korten AE, Henderson AS: The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatr Scand 1987; 76:465-479Crossref, Medline, Google Scholar
11. Jorm AF, van Duijn CM, Chandra V, Fratiglioni L, Graves AB, Heyman A, Kokmen E, Kondo K, Mortimer JA, Rocca WA (EURODEM Risk Factors Research Group): Psychiatric history and related exposures as risk factors for Alzheimer’s disease: a collaborative re-analysis of case-control studies. Int J Epidemiol 1991; 20(suppl 2):S43-S47Google Scholar



