Cross-National Comparisons of Seafood Consumption and Rates of Bipolar Disorders
Abstract
OBJECTIVE: The authors sought to determine if greater seafood consumption, a measure of omega-3 fatty acid intake, is associated with lower prevalence rates of bipolar disorder in community samples. METHOD: Lifetime prevalence rates in various countries for bipolar I disorder, bipolar II disorder, bipolar spectrum disorder, and schizophrenia were identified from population-based epidemiological studies that used similar methods. These epidemiological studies used structured diagnostic interviews with similar diagnostic criteria and were population based with large sample sizes. Simple linear and nonlinear regression analyses were used to compare these prevalence data to differences in apparent seafood consumption, an economic measure of disappearance of seafood from the economy. RESULTS: Simple exponential decay regressions showed that greater seafood consumption predicted lower lifetime prevalence rates of bipolar I disorder, bipolar II disorder, and bipolar spectrum disorder. Bipolar II disorder and bipolar spectrum disorder had an apparent vulnerability threshold below 50 lb of seafood/person/year. The absence of a correlation between lifetime prevalence rates of schizophrenia and seafood consumption suggests a specificity to affective disorders. CONCLUSIONS: These data describe a robust correlational relationship between greater seafood consumption and lower prevalence rates of bipolar disorders. These data provide a cross-national context for understanding ongoing clinical intervention trials of omega-3 fatty acids in bipolar disorders.
With the exception of periods of extreme famine, little is known about how the insufficient intake of nutrients that affect neuronal functioning impact the lifetime prevalence rates of psychiatric disorders (1–3). Prior studies that have compared ecological and cross-national differences in dietary fat (4) and seafood intake (5) with prevalence rates of cardiovascular disease have stimulated research that led to the dietary recommendations of the American Heart Association for the prevention of cardiovascular diseases (6). In a similar way, the identification of nutritional factors that correlate with prevalence rates of psychiatric disorders might also provide new strategies for treatment and prevention. Omega-3 essential fatty acids, specifically docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), are important candidates in the study of nutritional insufficiencies that may increase the risk of suffering psychiatric disorders. DHA in particular is selectively concentrated in synaptic membranes, comprising 20% of the phospholipid fatty acids, but this and other omega-3 fatty acids cannot be made de novo (7). Adequate dietary intakes of DHA are also clearly important for optimal neuronal development and function during infancy (8, 9). In an in vivo study, addition of DHA and arachidonic acid to infant formulas for 18 days nearly doubled concentrations of serotonin in the frontal cortex of infant piglets (10). Optimal synaptic membrane concentrations of DHA appear to be necessary to several mechanisms implicated in the pathophysiology of bipolar disorders, including phosphoinositol turnover (11, 12). Seafood and fish are rich dietary sources of both EPA and DHA.
Several studies support the hypothesis (13) that lower dietary intakes of seafood are related to higher prevalence rates of affective disorders. Hibbeln (14) found that greater seafood consumption was related to lower lifetime prevalence rates of major depression across nine countries (r=–0.84, p<0.005). Frequent fish consumption, twice a week or more, was an independent factor for a reduced risk for depressive symptoms (odds ratio=0.63) and suicidal thinking (odds ratio=0.57) in a restricted geographical region within a single country (15). Consistent with these findings, several authors have also reported lower plasma concentrations of EPA or DHA among depressed subjects compared with healthy subjects (16, 17). One placebo-controlled study of patients with bipolar disorder treated with omega-3 supplements reported a marked reduction in the number of severe affective episodes and a reduction in depressive symptoms over 4 months of treatment (18). On the basis of these findings, we postulated that lower lifetime prevalence rates of bipolar disorders would occur in countries with greater rates of seafood consumption. We tested this hypothesis by examining the epidemiological data on lifetime prevalence rates for bipolar disorders, using schizophrenia as a control condition, in various countries with differing rates of seafood consumption.
Method
Data on the prevalence rates of bipolar disorders and schizophrenia were obtained from published epidemiological studies. We used the Cross-National Collaborative Group epidemiological study of 10 countries (19, 20) and located other studies using MEDLINE and PsychInfo internet databases. Criteria for including studies in this analysis were 1) community samples with a clearly defined sample population, 2) a large sample size, 3) an age range between 18 and 64 years, and 4) appropriate sampling methods and use of structured diagnostic instruments. Thus, all studies had designs comparable in quality to the Epidemiologic Catchment Area study.
The Cross-National Collaborative Group included rates of bipolar I disorder for six countries: the United States, Canada, Puerto Rico, Taiwan, Korea, and New Zealand (20). Data from studies that met inclusion criteria were also available for Germany (21), Italy (22), Israel (23), Iceland (24), and Switzerland (25, 26). All studies used the National Institute of Mental Health Diagnostic Interview Schedule (DIS), version III (27), with the exception of Switzerland and Israel, which used the SPIKE (28) and Schedule for Affective Disorders and Schizophrenia (29), respectively. Bipolar II disorder rates were not available for Italy, Switzerland, Israel, or Iceland. A study of prevalence rates for bipolar II disorder in Hungary met inclusion criteria for our analysis (30). A Norway study (31) reported lifetime prevalence rates for all bipolar disorders, met inclusion criteria, and used the DIS but did not report diagnostic subcategories. The data from Norway were compared with other countries by summing the prevalence data for each subdiagnosis and defined as all bipolar disorders. Lifetime prevalence rates of schizophrenia, the control condition, were obtained from the Cross-National Collaborative Study for the United States, Germany, Canada, Puerto Rico, Taiwan, Korea, and New Zealand. Data from Spain (32), Israel (23), Iceland (24), Australia (24), United Kingdom (33), Greece (34), and Hong Kong (35) were also added to the analysis of lifetime prevalence rates of schizophrenia. All lifetime prevalence rates are in cases per 100,000 population.
Data describing national seafood consumption was obtained from a single source document compiled by the National Marine Fisheries Service and the Food and Agriculture Organization of the World Health Organization (36). The rates of seafood consumption appeared fairly consistent across the decade when the psychiatric prevalence studies were conducted (35). Apparent seafood consumption (lb/person/year) is a measure of disappearance of seafood from the economy per year and is calculated as total catch plus imports minus exports.
The lifetime prevalence rates (number per 100,000) of bipolar disorders and schizophrenia obtained from the Cross-National Collaborative Group were standardized at each site, and a design weight was calculated for each subject, stratified for age and sex. Prevalence rates weighted in this manner provided estimates as if each site had the same age and sex distribution. The standardization was done according to previously described methods (37). A few sites that were not part of the Cross-National group analysis (Germany, Hungary, Switzerland, Spain, Israel, and Iceland) could not be weighted for age and gender distribution because primary data were not available. Socioeconomic status and educational level were not considered in this analysis, since these variables are more difficult to define and compare in cross-national analyses. Only the 18–64-year-old age group from the Cross-National Collaborative Group was included in this analysis. The female-to-male ratio was approximately equal at all sites, with men having slightly higher rates than women in Canada, Puerto Rico, Korea, and New Zealand. The mean age at onset ranged from 18 years in the United States to 27 years in Puerto Rico. Age ranges for the additional samples were as follows: Hungary=18–64, Spain=17–65, Switzerland=19–34, Germany=18–54, Italy=18 and older, Israel=24–33, and Iceland=55–58 (birth cohort).
In the primary analysis, a linear univariate correlation compared the lifetime prevalence rates of bipolar I disorder, bipolar II disorder, bipolar spectrum disorder, and schizophrenia to the rates of apparent seafood consumption across countries. Pearson’s product-moment correlations were used to compare yearly seafood consumption and lifetime prevalence rates with simple linear and nonlinear models (Statview, SAS Institute, Cary, N.C.). Progressive empirical modeling estimated the best curve fitting for the nonlinear regression models (Sigma Plot, SPSS Science, Chicago, IL).
Results
As seen in Figure 1, the lifetime prevalence rate for bipolar spectrum disorder varied across 12 countries from 0.2 in Iceland to 6.5 in Germany. Rates for the intermediate countries, in order of increasing prevalence, were as follows: Taiwan=0.4, Korea=0.5, Puerto Rico=0.9, Canada=1.1, New Zealand=2.4, Israel=2.6, the United States=3.0, Italy=3.4, Switzerland=5.1, and Hungary=5.5. The lifetime prevalence rate for bipolar I disorder varied across 11 countries from 0.3 in Taiwan to 2.6 in Israel. Rates for the intermediate countries, in order of increasing prevalence, were as follows: Iceland=0.4, Korea=0.4, Canada=0.6, Puerto Rico=0.6, United States=0.9, Switzerland=1.3, Germany=1.4, New Zealand=1.5, and Italy=1.7. The lifetime prevalence rate for bipolar II disorder varied across eight countries from 0.1 in Taiwan to 2.0 in Hungary. Rates for the intermediate countries, in order of increasing prevalence, were as follows: Puerto Rico=0.2, Korea=0.2, Germany=0.4, United States=0.5, Canada=0.5, and New Zealand=1.0. For schizophrenia, the lifetime prevalence rate varied across 14 countries as follows: Hong Kong=0.1, Greece=0.2, Korea=0.3, Taiwan=0.3, Iceland=0.3, New Zealand=0.3, United Kingdom=0.4, Australia=0.5, Germany=0.6, Canada=0.6, Israel=0.7, United States=1.3, Puerto Rico=1.6, and Spain=1.7.
In simple linear regression models, higher national seafood consumption predicted lower prevalence rates of bipolar spectrum disorder (r=–0.67, df=10, p=0.02), bipolar I disorder (r=–0.52, df=9, p=0.09), and bipolar II disorder (r=–0.70, df=6, p=0.04). An examination of the residual plots of these findings suggested that nonlinear regressions would better describe the relationship between seafood consumption and rates of bipolar disorders. According to logarithmic regression models, greater seafood consumption predicted lower prevalence rates of bipolar I disorder (r=–0.60; r2=0.36, p<0.02), bipolar II disorder (r=–0.87; r2=0.76, p<0.0009), and bipolar spectrum disorder (r=–0.80; r2=0.64, p<0.0003). The best curve fitting came from a simple exponential decay regression (y=a × exp–bx); greater seafood consumption predicted lower rates of bipolar I disorder (r=–0.63; r2=0.40, p<0.04), bipolar II disorder (r=–0.89; r2=0.78, p<0.004), and bipolar spectrum disorder (r=–0.85; r2=0.72, p<0.0004). When defined as all bipolar disorders (i.e., all diagnostic subcategories summed), linear regression results (r=–0.74; r2=–0.54, p<0.0001) and exponential decay regression results (r=–0.85; r2=0.72, p<0.0001) remained significant. Exclusion of Iceland improved the strength of the relationship, per exponential decay regression, between seafood consumption and lifetime prevalence rate of bipolar II disorder (r=–0.91; r2=0.82, p<0.002) and did not significantly alter the results for bipolar I disorder (r=–0.59; r2=0.36, p<0.07) and bipolar spectrum disorder (r=–0.83; r2=0.68, p<0.002). There were no correlational relationships between lifetime prevalence rates of schizophrenia compared with any of the bipolar disorders across countries. Seafood consumption did not predict prevalence rates of schizophrenia in either linear or nonlinear models.
Discussion
These data indicate that greater rates of seafood consumption are associated with lower lifetime prevalence rates of bipolar I disorder, bipolar II disorder, and bipolar spectrum disorder. These findings do not demonstrate a causal relationship, but they are consistent with the hypothesis that an insufficient dietary intake of omega-3 essential fatty acids increases the risk of affective disorders (13). These findings are also consistent with the results of a double-blind, placebo-controlled trial that reported a reduction in the number of severe affective episodes and a reduction in depression scores in bipolar patients receiving 9.6 g/day of EPA and DHA (18). Bipolar II disorder is recognized to have prominent depressive symptoms (26). The strongest correlation was found between bipolar II disorder and seafood and fish consumption, which is consistent with the observation that omega-3 fatty acids are more effective in reducing depressive symptoms than manic symptoms (38). These results are also consistent with a prior cross-national study reporting a protective association between seafood consumption and major depression (14), with a study of fish intake and depressive symptoms in Finland (15), and with studies reporting lower tissue compositions of EPA and DHA among patients with major depression (16, 17). Given the direct tissue compositional data on seafood consumption and interventional treatment response of bipolar patients to omega-3 fatty acids (18), it seems likely that the biologically active components in seafood are EPA and DHA. However, other components in seafood cannot be ruled out. The biological plausibility of these findings is underscored by the observation that nearly every mechanism that has been implicated in pathophysiology of bipolar affective disorder (39) is affected by the tissue composition of essential fatty acids (12, 40).
There was no significant relationship between prevalence rates of schizophrenia and seafood consumption across countries. This result is consistent with a previous report of no cross-national association between seafood fat intake and either prevalence rates or outcome measures of schizophrenia (41). Recent treatment studies have, however, reported efficacy for EPA in both psychotic and depressive symptoms among severely ill schizophrenic patients early in their course of illness (42). In contrast, Fenton et al. (43) found no efficacy among chronically ill schizophrenia patients already being treated with optimal pharmacological therapies. Together, these data suggest that affective disorders may be more selectively responsive to omega-3 fatty acid status than schizophrenia.
Because bipolar disorders have a low prevalence, a variety of risk factors could not be examined. A small number of studies did not adjust for potential differences in age and gender distributions. In this study, we did not control for low socioeconomic status, rural/urban ratios, marital status, alcoholism, or smoking, and we did not have assessments of family history; all are all well known risk factors that predict the onset of bipolar disorders. An additional concern is that variability in the definition and diagnosis of bipolar spectrum disorder and prevalence data across countries may vary by diagnostic instrument. A further limitation of this study was that it could not determine if low seafood consumption increased lifetime risk for bipolar disorders because of effects solely in adulthood or as a result of nutritional insufficiency in early neurological development or both. Recent studies of gestational famine have identified that nutrient deficiencies during the second and third trimester specifically increase the risk of the development of affective disorders for the child (3). Neurodevelopmental impairment has been identified as a risk factor for later affective disorder (44).
Nonlinear regressions best described the relationships between apparent seafood intake and lifetime prevalence rates of bipolar disorders. One factor contributing to this nonlinear ecological relationship may be the nonlinear relationship between the dietary intake of essential fatty acids and the resulting tissue concentrations (45). A second possibility may be a threshold for biological vulnerability. Bipolar spectrum disorder showed the most clearly defined relationship with seafood consumption rates, with a threshold for increased bipolar vulnerability below the seafood consumption rate of approximately 50 lb per person per year (approximately 1–1.5 lb per person per week). We note that this rate of apparent seafood consumption represents total disappearance of seafood and fish products from the economy and not direct dietary intake. The level of 50 lb per person per year is at most approximately 300 g of seafood per person per day. Assuming that 100 grams of seafood contains 1 g of EPA plus DHA (U.S. Department of Agriculture food tables), this level corresponds to a maximum consumption of 3 g of EPA plus DHA per person per day. The U.S. Food and Drug Administration (FDA) states that 3 g of EPA plus DHA per day is generally recognized as safe (see FDA Code of Federal Regulations documents 21 CFR 172.860 and 21 CFR 184.1472 at http://www.fda.gov). We note that the most precipitous rise in prevalence rates for the bipolar disorders generally occurs in countries having a seafood consumption of less than 50 lb per person, which is well within the levels of consumption of EPA and DHA recognized by the FDA as safe.
Received April 4, 2001; revision received Dec. 7, 2001; accepted Sept. 5, 2003. From the New York State Psychiatric Institute, Columbia University College of Physicians and Surgeons, New York; and the National Institute on Alcohol Abuse and Alcoholism. Address reprint requests to Dr. Hibbeln, Laboratory of Membrane Biochemistry and Biophysics, NIAAA/NIH, 12420 Parklawn Dr., Room 1-14, Rockville, MD 20892; [email protected] (e-mail).
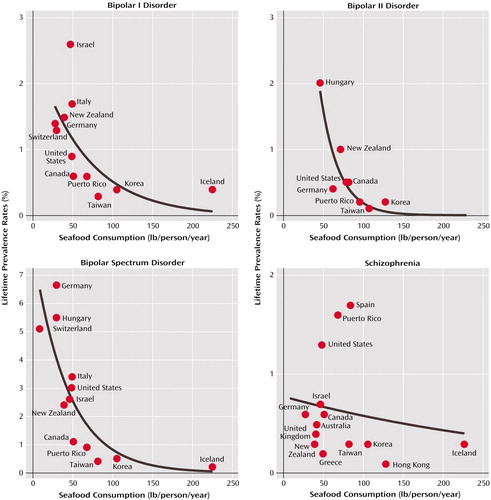
Figure 1. Cross-National Comparison of the Relationship Between Seafood Consumption and Lifetime Prevalence Rates of Bipolar Disorders and Schizophreniaa
aPsychopathology data obtained from population-based epidemiological studies that used structured diagnostic interviews and similar criteria for bipolar I disorder (N=11 countries), bipolar II disorder (N=8), bipolar spectrum disorder (N=12), and schizophrenia (N=14). A simple exponential decay equation (y=a × exp –bx) best described the relationship differences for bipolar I disorder (r=–0.63; r2=0.40, p<0.04), bipolar II disorder (r=–0.89; r2=0.78, p<0.004), and bipolar spectrum disorder (r=–0.85; r2=0.72, p<0.0004). Exclusion of data from Iceland did not significantly alter the results for bipolar I disorder (r=–0.59; r2=0.36, p<0.07) or bipolar spectrum disorder (r=–0.83; r2=0.68, p<0.002) and improved the results for bipolar II disorder (r=–0.91; r2=0.82, p<0.002).
1. Stein Z, Susser M: Effects of early nutrition on neurological and mental competence in human beings. Psychol Med 1985; 15:717–726Crossref, Medline, Google Scholar
2. Susser ES, Lin SP: Schizophrenia after prenatal exposure to the Dutch Hunger Winter of 1944–1945. Arch Gen Psychiatry 1992; 49:983–988Crossref, Medline, Google Scholar
3. Brown AS, van Os J, Driessens C, Hoek HW, Susser ES: Further evidence of relation between prenatal famine and major affective disorder. Am J Psychiatry 2000; 157:190–195Link, Google Scholar
4. Keys A: Coronary heart disease in seven countries (1970). Nutrition 1997; 13:250–252Crossref, Medline, Google Scholar
5. Bang HO, Dyerberg J: Fatty acid pattern and ischaemic heart disease (letter). Lancet 1987; 1:633Crossref, Medline, Google Scholar
6. Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, Erdman JW Jr, Kris-Etherton P, Goldberg IJ, Kotchen TA, Lichtenstein AH, Mitch WE, Mullis R, Robinson K, Wylie-Rosett J, St Jeor S, Suttie J, Tribble DL, Bazzarre TL: AHA dietary guidelines: revision 2000: a statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation 2000; 102:2284–2299Crossref, Medline, Google Scholar
7. Salem N Jr, Kim HY, Yergey JA: Docosahexaenoic acid: membrane function and metabolism, in Health Effects of Polyunsaturated Seafoods. Edited by Simopoulos A, Kifer RR, Martin R. New York, Academic Press, 1986, pp 263–317Google Scholar
8. Birch EE, Garfield S, Hoffman DR, Uauy R, Birch DG: A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev Med Child Neurol 2000; 42:174–181Crossref, Medline, Google Scholar
9. Willatts P, Forsyth JS, DiModugno MK, Varma S, Colvin M: Effect of long-chain polyunsaturated fatty acids in infant formula on problem solving at 10 months of age. Lancet 1998; 352:688–691Crossref, Medline, Google Scholar
10. de la Presa Owens S, Innis SM: Docosahexaenoic and arachidonic acid prevent a decrease in dopaminergic and serotoninergic neurotransmitters in frontal cortex caused by a linoleic and alpha-linolenic acid deficient diet in formula-fed piglets. J Nutr 1999; 129:2088–2093Medline, Google Scholar
11. Hibbeln JR, Linnoila M, Umhau JC, Rawlings R, George DT, Salem N Jr: Essential fatty acids predict metabolites of serotonin and dopamine in cerebrospinal fluid among healthy control subjects, and early- and late-onset alcoholics. Biol Psychiatry 1998; 44:235–242Crossref, Medline, Google Scholar
12. Stoll AL, Locke CA, Marangell LB, Severus WE: Omega-3 fatty acids and bipolar disorder: a review. Prostaglandins Leukot Essent Fatty Acids 1999; 60:329–337Crossref, Medline, Google Scholar
13. Hibbeln JR, Salem N Jr: Dietary polyunsaturated fatty acids and depression: when cholesterol does not satisfy. Am J Clin Nutr 1995; 62:1–9Crossref, Medline, Google Scholar
14. Hibbeln JR: Fish consumption and major depression (letter). Lancet 1998; 351:1213Crossref, Medline, Google Scholar
15. Tanskanen A, Hibbeln JR, Hintikka J, Haatainen K, Honkalampi K, Viinamäki H: Fish consumption, depression, and suicidality in a general population. Arch Gen Psychiatry 2001; 58:512–513Crossref, Medline, Google Scholar
16. Edwards R, Peet M, Shay J, Horrobin D: Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord 1998; 48:149–155Crossref, Medline, Google Scholar
17. Maes M, Smith R, Christophe A, Cosyns P, Desnyder R, Meltzer H: Fatty acid composition in major depression: decreased omega 3 fractions in cholesteryl esters and increased C20: 4 omega 6/C20:5 omega 3 ratio in cholesteryl esters and phospholipids. J Affect Disord 1996; 38:35–46Crossref, Medline, Google Scholar
18. Stoll AL, Severus WE, Freeman MP, Rueter S, Zboyan HA, Diamond E, Cress KK, Marangell LB: Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo controlled trial. Arch Gen Psychiatry 1999; 56:407–412Crossref, Medline, Google Scholar
19. Robins LN, Regier DA (eds): Psychiatric Disorders in America: The Epidemiologic Catchment Area Study. New York, Free Press, 1991Google Scholar
20. Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, Joyce PR, Karam EG, Lee CK, Lellouch J, Lepine JP, Newman SC, Rubio-Stipec M, Wells JE, Wickramaratne PJ, Wittchen H, Yeh EK: Cross-national epidemiology of major depression and bipolar disorder. JAMA 1996; 276:293–299Crossref, Medline, Google Scholar
21. Wittchen HU, Nelson CB, Lachner G: Prevalence of mental disorders and psychosocial impairments in adolescents and young adults. Psychol Med 1998; 28:109–126Crossref, Medline, Google Scholar
22. Faravelli C, Salvatori S, Galassi F, Aiazzi L, Drei C, Cabras P: Epidemiology of somatoform disorders: a community survey in Florence. Soc Psychiatry Psychiatr Epidemiol 1997; 32:24–29Crossref, Medline, Google Scholar
23. Levav I, Kohn R, Dohrenwend BP, Shrout PE, Skodol AE, Schwartz S, Link BG, Naveh G: An epidemiological study of mental disorders in a 10-year cohort of young adults in Israel. Psychol Med 1993; 23:691–707Crossref, Medline, Google Scholar
24. Stefansson JG, Lindal E, Bjornsson JK, Guomundsdottir A: Lifetime prevalence of specific mental disorders among people born in Iceland in 1931. Acta Psychiatr Scand 1991; 84:142–149Crossref, Medline, Google Scholar
25. Angst J: The emerging epidemiology of hypomania and bipolar II disorder. J Affect Disord 1998; 50:143–151Crossref, Medline, Google Scholar
26. Angst J: [Epidemiology of the bipolar spectrum]. Encephale 1995; 21(special number 6):37–42 (French)Google Scholar
27. Robins LN, Helzer JE, Croughan J, Ratcliff KS: The National Institute of Mental Health Diagnostic Interview Schedule: its history, characteristics, and validity. Arch Gen Psychiatry 1981; 38:381–389Crossref, Medline, Google Scholar
28. Angst J, Dobler-Mikola A, Binder J: The Zurich study—a prospective epidemiological study of depressive, neurotic and psychosomatic syndromes, I: problem, methodology. Eur Arch Psychiatry Neurol Sci 1984; 234:13–20Crossref, Medline, Google Scholar
29. Endicott J, Spitzer RL: Use of the Research Diagnostic Criteria and the Schedule for Affective Disorders and Schizophrenia to study affective disorders. Am J Psychiatry 1979; 136:52–56Link, Google Scholar
30. Szadoczky E, Papp Z, Vitrai J, Rihmer Z, Furedi J: The prevalence of major depressive and bipolar disorders in Hungary: results from a national epidemiologic survey. J Affect Disord 1998; 50:153–162Crossref, Medline, Google Scholar
31. Kringlen E, Torgersen S, Cramer V: A Norwegian psychiatric epidemiological study. Am J Psychiatry 2001; 158:1091–1098Link, Google Scholar
32. Vazquez-Barquero JL, Diez-Manrique JF, Pena C, Aldama J, Samaniego Rodriguez C, Menendez Arango J, Mirapeix C: A community mental health survey in Cantabria: a general description of morbidity. Psychol Med 1987; 17:227–241Crossref, Medline, Google Scholar
33. Bamrah JS, Freeman HL, Goldberg DP: Epidemiology of schizophrenia in Salford, 1974–84: changes in an urban community over ten years. Br J Psychiatry 1991; 159:802–810Crossref, Medline, Google Scholar
34. Mavreas VG, Beis A, Mouyias A, Rigoni F, Lyketsos GC: Prevalence of psychiatric disorders in Athens: a community study. Soc Psychiatry 1986; 21:172–181Crossref, Medline, Google Scholar
35. Chen C, Wong J, Lee N, Chan-Ho M, Lau JT, Fung M: The Shatin community mental health survey in Hong Kong II: major findings. Arch Gen Psychiatry 1993; 50:125–133Crossref, Medline, Google Scholar
36. World Health Organization: Fish and Fishery Products: World Apparent Consumption Based on Food Balance Sheets (1961–1993). Rome, WHO, Food and Agriculture Organization, 1996Google Scholar
37. Breslow NE, Day NE: Statistical Methods in Cancer Research, vol II: The Design and Analysis of Cohort Studies. Lyon, France, International Agency for Cancer Research, 1987Google Scholar
38. Su KP, Shen WW, Huang SY: Are omega 3 fatty acids beneficial in depression but not mania? Arch Gen Psychiatry 2000; 57:716–717Crossref, Medline, Google Scholar
39. Manji HK, Lenox RH: Signaling: cellular insights into the pathophysiology of bipolar disorder. Biol Psychiatry 2000; 48:518–530Crossref, Medline, Google Scholar
40. Hibbeln JR, Umhau JC, George DT, Shoaf SE, Linnoila M, Salem N Jr: Plasma total cholesterol concentrations do not predict cerebrospinal fluid neurotransmitter metabolites: implications for the biophysical role of highly unsaturated fatty acids. Am J Clin Nutr 2000; 71:331S-338SMedline, Google Scholar
41. Christensen O, Christensen E: Fat consumption and schizophrenia. Acta Psychiatr Scand 1988; 78:587–591Crossref, Medline, Google Scholar
42. Peet M, Horrobin DF (E-E Multicentre Study Group): A dose-ranging exploratory study of the effects of ethyl-eicosapentaenoate in patients with persistent schizophrenic symptoms. J Psychiatr Res 2002; 36:7–18Crossref, Medline, Google Scholar
43. Fenton WS, Dickerson F, Boronow J, Hibbeln JR, Knable M: A placebo-controlled trial of omega-3 fatty acid (ethyl eicosapentaenoic acid) supplementation for residual symptoms and cognitive impairment in schizophrenia. Am J Psychiatry 2001; 158:2071–2074Link, Google Scholar
44. van Os J, Jones P, Lewis G, Wadsworth M, Murray R: Developmental precursors of affective illness in a general population birth cohort. Arch Gen Psychiatry 1997; 54:625–631Crossref, Medline, Google Scholar
45. Lands WEM, Libelt B, Morris A, Kramer NC, Prewitt TE, Bowen P, Schmeisser D, Davidson MH, Burns JH: Maintenance of lower proportions of (n-6) eicosanoid precursors in phospholipids of human plasma in response to added dietary (n-3) fatty acids. Biochim Biophys Acta 1992; 1180:147–162Crossref, Medline, Google Scholar



