Progression of Subcortical Ischemic Disease From Vascular Depression to Vascular Dementia
In this case conference, we describe an elderly woman with recurrent major depression and subcortical ischemic disease who was seen for treatment. During the course of treatment, over the next several years, she experienced a new onset of congestive heart failure and developed cognitive deficits. Despite these confounding problems, her depression was successfully treated. This report illustrates 1) the challenges of medical and cognitive problems that often co-occur with depression in the elderly, 2) that with persistence, antidepressant therapy in this population can be effective, and 3) that these problems may often have a common pathophysiology—vascular disease—which necessitates thorough evaluation and treatment of more than mere depression.
Case Report
Ms. A was a 78-year-old widowed white woman whose daughters brought her to our mood disorders clinic for evaluation of recurrent major depression.
History
Ms. A’s chief complaint was “I don’t feel like doing anything anymore.” Over the previous 6 months, Ms. A had become increasingly depressed, dependent on her daughters, and more physically frail. She was not motivated to perform her usual activities. She reported a depressed mood, frequent crying spells, poor sleep, with trouble falling asleep, as well as frequent awakenings during the night, a low energy level, and difficulty concentrating. She reported no suicidal ideation or psychotic symptoms. She was taking paroxetine, 20 mg/day, at the time of her initial evaluation.
Ms. A had a history of depression dating back approximately 13 years, which occurred in the context of marital discord and “a spell” that was later diagnosed as a left internal capsule lacunar infarct. During the course of that episode, she had failed to respond to several medications and ultimately received a course of 14 sessions of ECT, to which she responded well. Her mood had been maintained with antidepressant medications, most recently sertraline, until 3 years previously, when her husband died. At that time, she developed a recurrence of depressive symptoms, along with a decreased capacity for self-care; this impairment was so significant that her daughters placed her in an assisted-living facility so that her needs could be better met. Her psychiatrist changed her medications to a combination of trimipramine and sertraline. One year later, or 2 years before Ms. A was seen for treatment, she had experienced a recurrence of severe depressive symptoms, was admitted to the hospital, and received a course of eight ECT treatments. She was given paroxetine, 20 mg at bedtime, which she continued to take over the next 2 years. During that hospitalization, a brain magnetic resonance imaging (MRI) scan revealed moderately severe subcortical white matter disease.
Ms. A’s medical history was significant for hypercholesterolemia, gastritis, gouty arthritis, and osteoporosis. Her medications included 40 mg of simvastatin at bedtime, 20 mg of paroxetine at bedtime, 300 mg b.i.d. of allopurinol, 150 mg b.i.d. of ranitidine, 0.625 mg/day of conjugated estrogen, and 500 mg/day of a calcium supplement.
Ms. A’s social history revealed that she was the youngest of four children. Her father had a stroke when she was 13; Ms. A cared for him until she her early 20s, when she married. She and her husband were married for 49 years and raised two daughters. She completed high school but never had regular employment outside of the home, and she never learned to drive.
Ms. A’s daughters noted that after their father died, “Mother fell apart.” They thought that the paroxetine had helped improve her depression to some extent, particularly her ability to manage her household affairs. However, they stated that she had “never been the same” since their father’s death.
A mental status examination revealed a melancholy older woman with little spontaneous movement. Her speech pattern had a low tone and a high latency. She generally had a flat affect but was sporadically tearful during the interview. She described her mood as “low.” Her psychomotor activity was diminished, and her thought processes were goal directed. She reported poor concentration. She reported no psychotic symptoms or suicidal or homicidal ideation. She scored 28 of 30 on the Mini-Mental State Examination (MMSE) (1); she incorrectly answered one question regarding the country she lived in and missed one question by spelling “world” backward.
Ms. A’s daughters brought her most recent results from laboratory testing from her general practitioner. They included normal results for blood chemistries, a CBC, and a thyroid profile, including levels of thyroid-stimulating hormone, vitamin B12, and folate.
Initial Treatment Plan
At her initial evaluation, Ms. A was diagnosed with recurrent severe major depression. She agreed to enroll in our mental health clinical research center, through which she received a cranial MRI scan. The initial plan was to taper her off paroxetine and initiate treatment with sertraline at a dose of 25 mg/day. In addition, Ms. A and her daughters were instructed to work with the assisted-living facility’s activities staff to identify pleasurable activities in which Ms. A could gradually increase her involvement.
Course of Treatment
Year 1
Over the next 3 months, Ms. A switched from paroxetine to sertraline, which was increased to 50 mg/day. She continued to experience depressive symptoms, so during the ensuing 6 months, her sertraline dose was further titrated to 100 mg/day. At this dose, she noted an improvement in her sleep and appetite. Her mood appeared brighter. However, she continued to experience little interest in activities, a diminished ability to experience pleasure in activities going on at the assisted-living facility, and low energy levels. At this point, sustained-release bupropion, 100 mg/day, was added to her sertraline dose.
Over the ensuing 2 to 3 months, halfway through her first year of treatment, Ms. A had mild improvement in both her mood and apathy symptoms. At her 9-month visit, she began to experience some weakness and shortness of breath when walking. She was diagnosed with congestive heart failure. Both long-acting diltiazem and digoxin were added to her medication regimen. Rather than changing her antidepressant medications, the decision was made to monitor her progress with sertraline and sustained-release bupropion while her medical problems were being treated.
Year 2
Six months later, at her 15-month visit, Ms. A’s daughters were concerned that she continued to spend much of her time in bed and refused to pursue activities suggested by her assisted-living facility. They also reported that she was confused at times during the day. They had taken her to her general practitioner, who could not find any acute medical problem to explain her symptoms. Upon examination, she reported a full range of depression symptoms and had an MMSE score of 16. Another course of ECT sessions was discussed, but Ms. A was not interested. Instead, at this time, the decision was made to taper her off the sertraline, discontinue the bupropion, and initiate extended-release venlafaxine. She started taking a dose of 37.5 mg/day of venlafaxine, and over the ensuing 2 weeks, her dose was increased to 75 mg/day.
Six weeks later, one of Ms. A’s daughters called the treating psychiatrist and reported, “Mother is a new woman.” Ms. A had begun to participate more in activities and was more interested in what was going on at the assisted-living facility and with her family. At her 18-month visit, Ms. A appeared in a brighter mood and reported no depressive symptoms. Despite this improvement in her depressive symptoms, during the interview, Ms. A had difficulty processing information. Her MMSE score was 21; she had errors regarding the year, the date, the day of the week, the month, the country, and the floor of building, spelled “world” in a convoluted manner (“DLROE”), recalled only one of three items, and had difficulty placing a sheet of paper on her lap.
Years 3 and 4
At Ms. A’s 24-month visit, her depression remained stable while she was taking extended-release venlafaxine. Her daughters noticed that she continued to be confused at times, with ongoing difficulties in keeping up with what was going on in the family. She did not comprehend everything that people were saying, which she blamed on poor hearing. Her treating psychiatrist maintained her status with extended-release venlafaxine and initiated donepezil, 5 mg at bedtime. Her dose was increased to 10 mg at bedtime 12 weeks later.
Six months later, Ms. A continued to be in remission of her depressive symptoms. In the interim, her general practitioner had noted her cognitive impairment and had initiated a course of monthly vitamin B12 injections. After 4 years of follow-up, Ms. A continued to do well in terms of her depressive symptoms. Her daughters noted an improved awareness of what was going on around her. Her MMSE score was 25, with errors regarding orientation and in following a three-step command.
Changes in Ms. A’s Cranial MRI
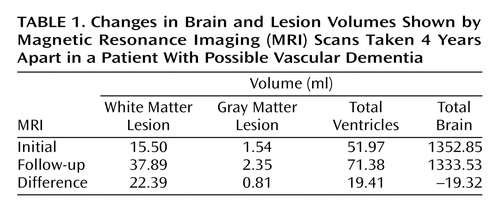
Because of her participation in the clinical research study, Ms. A received an initial cranial MRI, then another MRI 4 years later (Figure 1). A semiautomated segmentation method (2) was used to measure total brain, ventricle, and lesion volumes. Ms. A demonstrated a striking increase in both gray and white matter lesion volumes over this period, while maintaining a relatively constant total brain volume (Table 1).
Discussion
Many features of late-life depression are illustrated by this case. Vascular depression is comorbid with medical illness, particularly cerebrovascular disease. Vascular risk factors contribute not only to the pathogenesis of depression but also complicate its treatment. Many older individuals with depression may be medically frail. Furthermore, progression of cerebrovascular disease may lead to clinically evident cognitive impairment. All of these issues are essential to consider in treating depressed elderly patients and are important for further refinement of the hypothesis of vascular depression.
However, these medical comorbidities do not lessen the effect of psychosocial factors. Ms. A’s depressive symptoms began after a stroke and in the context of stress. The origin of depression in this population is thus multifactorial and may manifest in many ways. For Ms. A, the most significant initial piece of her medical history was a stroke. As seen in many other elderly individuals, her social stressors were the death of a spouse and placement in a supervised assisted-living facility.
Vascular Disease and Depression
Cerebrovascular disease is commonly seen in cranial MRI of depressed elders. The presence of depression in the context of vascular disease has been labeled as vascular depression (3–5). This individual had extensive cerebrovascular disease, exhibiting not only a stroke but also extensive subcortical ischemic disease (Figure 1). Such subcortical ischemic disease has been linked not only to depression (4, 6–8) but also to dementia (9, 10) and falling (11). The risk factors for subcortical ischemic disease include the same risk factors as for stroke: hypertension, diabetes, hyperlipidemia, smoking, and coronary artery disease (12–15). In a large-scale study of 240 subjects, Awad et al. (16) reported that cerebrovascular risk factors, such as hypertension and coronary artery disease, were related to severity of lesions (80% of those with grade-3 or grade-4 lesions had hypertension). Inzitari et al. (17) found that 64% of patients with leukoencephalopathy had hypertension, compared with 34% of comparison subjects. Although some studies of smaller groups did not show a significant relationship between subcortical ischemic disease and these cerebrovascular risk factors, the general consensus is that these changes are more severe and relevant in those with cerebrovascular risk factors. Of note, these cerebrovascular risk factors do not account for all cases of subcortical ischemic disease; there is also a familial component to the development of these changes. As much as 50% of these changes may develop from genetic factors (18). Genetic contributions are seen in similar disorders, such as cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (19). In Ms. A’s case, hyperlipidemia was clearly a risk factor, but she was adequately treated.
Antidepressant Therapy and Medical Illness in the Elderly
The selection of switching to another selective serotonin reuptake inhibitor (SSRI) when the first SSRI was ineffective was based on evidence that such a change can sometimes work. In this case, the decision was also driven by a good previous response to sertraline. When faced with a patient who does not respond to an SSRI, many clinicians may preferentially switch a patient to an antidepressant of a different class (20). Despite this common practice, there is some evidence that patients who do not respond to or cannot tolerate one SSRI may respond to or tolerate another SSRI (21–24). The SSRI selected in this instance (sertraline) additionally has a proven record of safety in elderly individuals with medical illness (25, 26). Other choices could have included switching to an antidepressant of a different class or augmentation with lithium. The choice of sustained-release bupropion as an augmenting agent was based on case reports suggesting that this approach is effective (27–29), but there is no evidence for this decision from well-designed, randomized controlled trials. A large trial (STAR*D; http://www.edc.gsph. pitt.edu/stard) is under way to test the efficacy of a variety of antidepressant treatment options, including this particular augmentation strategy.
The development of other medical problems may also complicate the diagnosis or management of depression. In this instance, Ms. A developed congestive heart failure, a disease common in the elderly that may mimic the symptoms of depression. Congestive heart failure can produce the fatigue, low energy levels, and sleep disturbances that also are symptoms of depression. Additionally, the presence of depression in individuals with congestive heart failure increases their chances of dying (30).
The choice of medication treatment in the context of medical illness must be tailored to an individual patient. First, drug-disease interactions must be considered. In this case, the presence of congestive heart failure influenced the choice of medications. Venlafaxine, which could increase blood pressure, may worsen congestive heart failure in theory, although there is no direct evidence for such an association. The fact that Ms. A’s congestive heart failure was well controlled at the time she was given this agent made it a reasonable choice in her individual case. Another factor to consider is drug-drug interactions. The use of diltiazem means that one cannot use drugs with potential interactions, such as lithium.
There is a nihilistic assumption that depression in the context of medical illness is difficult to treat. This assumption is not warranted, and an article in the Cochrane Database is particularly relevant (31). We searched for all relevant randomized trials comparing any antidepressant drug with placebo or no treatment in patients who had been diagnosed as depressed by any criterion and had a specified physical disorder (e.g., cancer, myocardial infarction). The main outcome measures of these studies were the numbers of individuals who recovered or improved by the end of the trial or the numbers of those who completed treatment. Eighteen studies were reviewed, which included 838 patients with a range of physical diseases. The patients treated with antidepressants were significantly more likely to improve than those given placebo or no treatment. We also found that the extent of recovery in the patients both with and without cardiac illness was similar. The recent SADHART trial regarding depression after myocardial infarctions (26) also suggests that antidepressants may be helpful in the treatment of major depression in the context of medical illness. This may be particularly true for individuals with a past history of depressive episodes. Thus, it is important to recognize and effectively treat depression in the context of medical illness.
The relative lack of evidence for the efficacy of antidepressant treatment in the elderly deserves comment. Clinicians caring for older depressed patients often must rely on the results of antidepressant trials in younger populations to inform their decisions. While there are small or open-label studies that support the use of antidepressants in the elderly, there have been few published large-scale, placebo-controlled, multicenter trials of antidepressant use in elderly groups. A Cochrane Database review discussed what data is currently available (32). Clearly, more trials of antidepressant use in elderly populations are required to establish safety and determine appropriate initial and maintenance dosing.
Finally, psychosocial interventions may also be helpful. Apathy is commonly seen in depressed elderly patients. To achieve normal functioning, many older adults benefit from a behavioral plan to resume pleasurable activities. For Ms. A, this plan involved working with the activities staff of her assisted-living facility. This demonstrates that in elderly patients, it is crucial to treat the whole patient. In this instance, treatment management involved antidepressant medication, behavioral activation, and attention to the treatment of medical conditions.
Depression and Cognitive Impairment
The risk of developing cognitive deficits increases with age and may be higher in depressed elders. Ms. A’s illness course during the second and third years of treatment indicates the possibility of pseudodementia or, a more recent term, “reversible dementia of depression.” A decrease in cognitive functioning in the context of depression is common in depressed elderly patients. Her partial, but not complete, recovery is also emblematic. Alexopoulos et al. (33), in their classic work on pseudodementia, showed that in these patients, cognition deteriorates over time and that the level of cognitive function may not return to normal. Irreversible dementia developed significantly more frequently in a depressed group with reversible dementia (43%) than in those with depression alone (12%) (33). The group with reversible dementia had a fourfold higher risk of developing dementia at follow-up than cognitively intact depressed subjects. Subcortical ischemic disease has also been associated with poor cognition (34) and is a known risk factor for the development of dementia (35).
We may compare the conceptualization of vascular depression with the evolution of our understanding of vascular dementia. Vascular dementia may be caused by multiple strokes but also by single strategic strokes, multiple lacunae, and hypoperfusive lesions, such as border zone infarcts and ischemic periventricular leukoencephalopathy (Binswanger’s disease). The choice of donepezil for treating this type of dementia is supported by both pilot (36, 37) and large recently published trials (38), which demonstrates the use of cholinesterase inhibitors in individuals with probable vascular dementia, although these studies do have limitations (39). Ms. A’s improvement in cognition with donepezil treatment is similar to that reported in the literature.
Conclusions: The Vascular Depression Hypothesis
As is seen in vascular dementia, vascular depression may result from a variety of cerebrovascular insults. The type of vascular depression seen here reflects the development of depression in the context of a stroke and with the progression of subcortical ischemic disease. Dementia occurring as a consequence of subcortical ischemic disease is called subcortical ischemic dementia. Since the disease process is likely similar for mood disorders, the term “subcortical ischemic depression” may be appropriate.
This case illustrates the intertwining course of depression with a progression of medical illness and cognitive deficits, demonstrating the complexity of this evolving relationship. Mood disturbances associated with subcortical ischemic disease may meet full criteria for major depression, bipolar disorder, or dysthymia. Less severe or chronic mood disturbances are likely associated with subcortical ischemia; data from the Cardiovascular Health Study (40, 41) clearly documents the relationship between minor symptoms of depression and subcortical ischemic disease. However, with the exception of ICD-defined minor depression, our current diagnostic nomenclature does not capture these other disturbances well.
It is important to note the considerable overlap in mood and cognitive symptoms in patients with subcortical ischemic disease. Patients with lacunar infarcts may exhibit a combination of mood, cognitive, and other symptoms. Patients with late-life depression often have executive dysfunction. Such dysfunction is associated with impairment in instrumental activities and functional disability, which may persist even after the mood symptoms have been successfully treated. As this case illustrates, over time these patients may develop dementia. This issue requires further research to determine if interventions may slow or prevent this deterioration. Just as in the case of vascular dementia, treatment of vascular risk factors may improve the prognosis. This hypothesis should be tested in future trials.
 |
Received Dec. 31, 2002; revision received July 3, 2003; accepted July 10, 2003. From the Department of Psychiatry and Behavioral Sciences, Duke University Medical Center. Address reprint requests to Dr. Steffens, Duke University Medical Center, DUMC 3903, Durham, NC 27710; [email protected] (e-mail). Supported by NIMH grants MH-60451 and MH-54846 and by a National Alliance for Research on Schizophrenia and Depression Young Investigator Award. The authors thank James R. MacFall, Ph.D., and Martha E. Payne, R.D., for development of the brain segmentation procedure and Denise Fetzer for image processing.
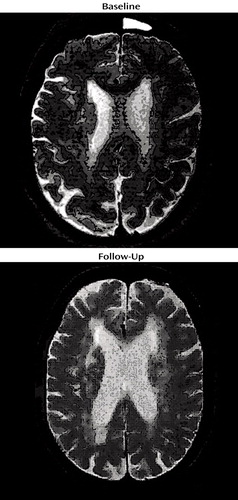
Figure 1. Serial Brain Magnetic Resonance Images Taken 4 Years Apart in a Patient With Possible Vascular Dementiaa
aThe upper image was T2-weighted. These images were taken near the same axial level, although the patient’s head was tilted differently. There is a visible difference in ventricle size and in the severity of subcortical hyperintense lesions.
1. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
2. Payne ME, Fetzer DL, MacFall JR, Provenzale JM, Byrum CE, Krishnan KRR: Development of a semi-automated method for quantification of MRI gray and white matter lesions in geriatric subjects. Psychiatry Res 2002; 115:63–77Crossref, Medline, Google Scholar
3. Krishnan KRR, McDonald WM: Arteriosclerotic depression. Med Hypotheses 1995; 44:111–115Crossref, Medline, Google Scholar
4. Krishnan KRR, Hays JC, Blazer DG: MRI-defined vascular depression. Am J Psychiatry 1997; 154:497–501Link, Google Scholar
5. Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M: “Vascular depression” hypothesis. Arch Gen Psychiatry 1997; 54:915–922Crossref, Medline, Google Scholar
6. Krishnan KRR, Goli V, Ellinwood EH, France RD, Blazer DZ, Nemeroff CB: Leukoencephalopathy in patients diagnosed as major depressive. Biol Psychiatry 1988; 1988; 23:519–522Google Scholar
7. Greenwald BS, Kramer-Ginsberg E, Krishnan KRR, Ashtari M, Aupperle PM, Patel M: MRI signal hyperintensities in geriatric depression. Am J Psychiatry 1996; 153:1212–1215Link, Google Scholar
8. Kumar A, Bilker W, Jin Z, Udupa J: Atrophy and high intensity lesions: complementary neurobiological mechanisms in late-life depression. Neuropsychopharmacology 2000; 22:264–274Crossref, Medline, Google Scholar
9. Looi JCL, Sachdev PS: Differentiation of vascular dementia from Alzheimer’s disease on neuropsychological tests. Neurology 1999; 53:670–678Crossref, Medline, Google Scholar
10. Roman GC, Royall DR: Executive control function: a rational basis for the diagnosis of vascular dementia. Alzheimer Dis Assoc Disord 1999; 13(suppl 3):S69-S80Google Scholar
11. Kerber KA, Enrietto JA, Jacobson KM, Baloh RW: Disequilibrium in older people: a prospective study. Neurology 1998; 51:574–580Crossref, Medline, Google Scholar
12. Longstreth WT Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O’Leary D, Fried L: Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people: the Cardiovascular Health Study. Stroke 1996; 27:1274–1282Crossref, Medline, Google Scholar
13. Fazekas F, Niederkor K, Schmidt R, Offenbacher H, Honner S, Bertha G, Lechner H: White matter signal abnormalities in normal individuals: correlation with carotid ultrasonography, cerebral blood flow measurements, and cerebrovascular risk factors. Stroke 1988; 19:1285–1288Crossref, Medline, Google Scholar
14. Ylikoski A, Erkinjuntti T, Raininko R, Sarna S, Sulkava R, Tilvis R: White matter hyperintensities on MRI in the neurologically nondiseased elderly: analysis of cohorts of consecutive subjects aged 55 to 85 years living at home. Stroke 1995; 26:1171–1177Crossref, Medline, Google Scholar
15. Sato R, Bryan RN, Fried LP: Neuroanatomic and functional correlates of depressed mood: the Cardiovascular Health Study. Am J Epidemiol 1999; 150:919–929Crossref, Medline, Google Scholar
16. Awad IA, Spetzler RF, Hodak JA, Awad CA, Carey R: Incidental subcortical lesions identified on magnetic resonance imaging in the elderly, I: correlation with age and cerebrovascular risk factors. Stroke 1986; 17:1084–1089Crossref, Medline, Google Scholar
17. Inzitari D, Diaz F, Fox A, Hachinski VC, Steingart A, Lau C, Donald A, Wade J, Mulic H, Merskey H: Vascular risk factors and leukoariosis. Arch Neurol 1987; 44:42–47Crossref, Medline, Google Scholar
18. Carmelli D, Reed T, DeCarli C: A bivariate genetic analysis of cerebral white matter hyperintensities and cognitive performance in elderly male twins. Neurobiol Aging 2002; 23:413–420Crossref, Medline, Google Scholar
19. Dichgans M: CADASIL: a monogenic condition causing stroke and subcortical vascular dementia. Cerebrovasc Dis 2002; 13(suppl 2):37–41Google Scholar
20. Fredman SJ, Fava M, Kienke AS, White CN, Nierenberg AA, Rosenbaum JF: Partial response, nonresponse, and relapse with selective serotonin reuptake inhibitors in major depression: a survey of current “next-step” practices. J Clin Psychiatry 2000; 61:403–408Crossref, Medline, Google Scholar
21. Brown WA, Harrison W: Are patients who are intolerant to one serotonin selective reuptake inhibitor intolerant to another? J Clin Psychiatry 1995; 56:30–34Medline, Google Scholar
22. Zarate CA, Kando JC, Tohen M, Weiss MK, Cole JO: Does intolerance or lack of response with fluoxetine predict the same will happen with sertraline? J Clin Psychiatry 1996; 57:67–71Medline, Google Scholar
23. Joffe RT, Levitt AJ, Sokolov ST, Young LT: Response to an open trial of a second SSRI in major depression. J Clin Psychiatry 1996; 58:236–237Google Scholar
24. Thase ME, Blomgren SL, Birkett MA, Apter JT, Tepner RG: Fluoxetine treatment of patients with major depressive disorder who failed initial treatment with sertraline. J Clin Psychiatry 1997; 58:16–21Crossref, Medline, Google Scholar
25. Krishnan KRR, Doraiswamy PM, Clary CM: Clinical and treatment response characteristics of late-life depression associated with vascular disease: a pooled analysis of two multicenter trials with sertraline. Prog Neuropsychopharmacol Biol Psychiatry 2001; 25:347–351Crossref, Medline, Google Scholar
26. Glassman AH, O’Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT, Krishnan KRR, van Zyl LT, Swenson JR, Finkel MS, Landau C, Shapiro PA, Pepine CJ, Mardekian J, Harrison WM: Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002; 288:701–709Crossref, Medline, Google Scholar
27. Bodkin JA, Lasser RA, Wines JD Jr, Gardner DM, Baldessarini RJ: Combining serotonin reuptake inhibitors and bupropion in partial responders to antidepressant monotherapy. J Clin Psychiatry 1997; 58:137–145Crossref, Medline, Google Scholar
28. Spier SA: Use of bupropion with SRIs and venlafaxine. Depress Anxiety 1998; 7:73–75Crossref, Medline, Google Scholar
29. Ramasubbu R: Treatment of resistant depression by adding noradrenergic agents to lithium augmentation of SSRIs. Ann Pharmacother 2002; 36:634–640Crossref, Medline, Google Scholar
30. Jiang W, Alexander J, Christopher E, Kuchibhatla M, Gaulden LH, Cuffe MS, Blazing MA, Davenport C, Califf RM, Krishnan RR, O’Connor CM: Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med 2001; 161:1849–1856Crossref, Medline, Google Scholar
31. Gill D, Hatcher S: Antidepressants for depression in medical illness. Cochrane Database Syst Rev 2000; (4):CD001312Google Scholar
32. Wilson K, Mottram P, Sivanranthan A, Nightingale A: Antidepressants versus placebo for the depressed elderly. Cochrane Database Syst Rev 2001; (2):CD000561Google Scholar
33. Alexopoulos GS, Meyers BS, Young RC, Mattis S, Kakuma T: The course of geriatric depression with “reversible dementia”: a controlled study. Am J Psychiatry 1993; 150:1693–1699Link, Google Scholar
34. Cannata AP, Alberoni M, Franceschi M, Mariana C: Frontal impairment in subcortical ischemic vascular dementia in comparison to Alzheimer’s disease. Dement Geriatr Cogn Disord 2002; 13:101–111Crossref, Medline, Google Scholar
35. Chui H: Dementia due to subcortical ischemic vascular disease. Clin Cornerstone 2001; 3:40–51Crossref, Medline, Google Scholar
36. Li Y, Meyer JS, Haque MA, Chowdhury MH, Hinh P, Quach M: Feasibility of vascular dementia treatment with cholinesterase inhibitors. Int J Geriatr Psychiatry 2002; 17:194–196Crossref, Medline, Google Scholar
37. Mendez MF, Younesi FL, Perryman KM: Use of donepezil for vascular dementia: preliminary clinical experience. J Neuropsychiatry Clin Neurosci 1999; 11:268–270Crossref, Medline, Google Scholar
38. Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Rao C, Damaraju V: Efficacy of galantamine in probable vascular dementia and Alzheimer’s disease combined with cerebrovascular disease: a randomised trial. Lancet 2002; 359:1283–1290Crossref, Medline, Google Scholar
39. Schneider LS: Galantamine for vascular dementia: some answers, some questions. Lancet 2002; 359:1265–1266Crossref, Medline, Google Scholar
40. Steffens DC, Helms MJ, Krishnan KRR, Burke GL: Cerebrovascular disease and depression symptoms in the Cardiovascular Health Study. Stroke 1999; 30:2159–2166Crossref, Medline, Google Scholar
41. Steffens DC, Krishnan KRR, Crump C, Burke GL: Cerebrovascular disease and evolution of depressive symptoms in the cardiovascular health study. Stroke 2002; 33:1636–1644Crossref, Medline, Google Scholar



