The Safety of Valproic Acid Use for Patients With Hepatitis C Infection
Abstract
OBJECTIVE: Valproic acid is frequently not recommended for patients with hepatic dysfunction. The authors evaluated the association between hepatitis C and alanine aminotransferase (ALT) values during valproic acid treatment. METHOD: ALT changes in 564 individuals beginning valproic acid treatment were examined. Changes among those with positive hepatitis C status were compared with changes among patients with positive hepatitis C status who were taking other psychotropic agents. RESULTS: ALT elevations with valproic acid were significantly greater among patients with positive hepatitis C status than those with negative or unknown status. Among patients with positive hepatitis C status, ALT increases did not differ significantly between valproic acid and other medications. CONCLUSIONS: Use of valproic acid may be possible for some patients with hepatitis C. ALT increases in seropositive patients may be partially related to chronic hepatitis infection. However, ALT levels should be closely monitored in all hepatitis C patients taking valproic acid.
Valproic acid is widely used to treat a variety of psychiatric illnesses (1). Although valproic acid is generally well tolerated, it frequently causes transient elevations in liver transaminases (2, 3). However, hepatotoxicity induced by valproic acid is a rare and potentially fatal adverse reaction (4–6). Concerns about hepatotoxicity have resulted in warnings that valproic acid should not be used for patients with significant hepatic dysfunction (7, 8).
The hepatitis C virus is one of the most common causes of chronic viral hepatitis (9). Once hepatitis C infection has been established, disease status is best evaluated by histologic biopsy (10, 11). Because serial liver biopsies are expensive and impractical, serum levels of alanine aminotransferase (ALT) are often followed in an attempt to assess ongoing liver damage (12).
Unfortunately, psychiatric disorders are highly prevalent in patients with hepatitis C (13, 14). However, concerns about potential valproic acid hepatotoxicity cause reluctance to use valproic acid for patients with comorbid hepatitis C, even when it would otherwise be the psychiatric treatment of choice. In order to examine the safety of valproic acid for patients with hepatitis C, we evaluated the association between valproic acid treatment and serum ALT changes in patients with and without the hepatitis C virus.
Method
Approval for this study of human subjects from the University of Washington and from the research committee of the Department of Veterans Affairs (VA) Puget Sound Health Care System was obtained. We used the VA Puget Sound Health Care System pharmacy and laboratory databases from 1994 through 2000 to find all patients meeting the following criteria: 1) a new valproic acid (or divalproex) prescription, 2) at least one baseline measurement of ALT within the 90 days before the beginning of treatment with valproic acid, 3) at least one follow-up ALT measurement within the 90 days after the beginning of valproic acid treatment, and 4) ongoing outpatient care at VA Puget Sound Health Care System as evidenced by at least one outpatient prescription filled in the 180 days both before and after the first prescription for valproic acid was filled. The study group included all patients who began treatment with valproic acid and was not limited to mental health patients. Diagnoses were obtained from ICD-9-CM codes contained within the computerized medical record.
The VA Puget Sound Health Care System laboratory database was used to determine hepatitis C virus status. Hepatitis C virus status was negative if the patient had a negative result on a hepatitis C virus enzyme immunoassay or if the patient had a positive hepatitis C enzyme immunoassay but a negative result on a recombinant immunoblot assay for hepatitis C. Individuals classified as having positive hepatitis C status had either hepatitis C antibodies or a positive result on a hepatitis C polymerase chain reaction test (PCR). Patients who had not been tested were categorized as having unknown hepatitis C status.
Maximum ALT values after the beginning of therapy were the outcome measure of interest. Normal serum ALT values in our laboratory are 40 U/liter or less. However, many of the subjects in this study group started treatment with high transaminase levels. To create clinically meaningful definitions of “elevated ALT level” that reflected both the absolute value and the change from baseline, we defined three categories of change in serum ALT levels, similar to what has been reported in previous work (15). A “marked elevation” was defined as a peak level 2.0 times the baseline value and at least 3.0 times the upper limit of normal; a “moderate elevation” was a peak level between 1.5 and 2.0 times baseline and above the upper limit of normal; and “no/minimal elevation” was a peak level less than 1.5 times the baseline value or within normal limits.
In order to distinguish between the hepatic effects of valproic acid and the underlying effects of ongoing hepatitis C infection, we also gathered ALT data for two comparison groups receiving active treatment who also had positive findings for hepatitis C. The first consisted of individuals with new antidepressant prescriptions (for tricyclics or serotonin reuptake inhibitors other than nefazodone) because these agents are commonly prescribed to patients with hepatitis C. The second group consisted of patients beginning treatment with lithium or gabapentin, who were chosen because these agents are frequently prescribed to psychiatric patients, but they are not metabolized in the liver and should not be associated with liver toxicity. All patients in these comparison groups were known to 1) have positive hepatitis C status and 2) meet all the other preceding criteria except that they had never been given a prescription for valproic acid according to computerized records.
Associations between hepatitis C status and demographic characteristics were tested with Fisher’s exact test, chi-square analysis, or one-way analysis of variance, as appropriate.
Results
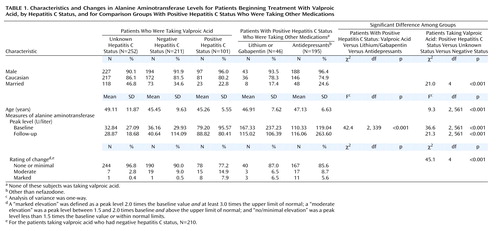
Among the patients with prescriptions for valproic acid, 564 were identified as meeting the study criteria; approximately 37% had negative findings for the hepatitis C virus, 18% had positive hepatitis C findings, and 45% had unknown status (Table 1). There were no significant associations between race or sex and hepatitis C status. However, among the patients who were taking valproic acid, those with positive hepatitis C status were significantly less likely to be married. There was also a significant difference among the mean ages of the groups. Individuals with unknown hepatitis C status were older than those with negative and positive status. The psychiatric diagnoses for the patients taking valproic acid included bipolar disorder, depression, psychosis, and posttraumatic stress disorder. Many patients had more than one of these diagnoses, making diagnostic specificity difficult. Of all the patients receiving valproic acid, only 43 (7.6%) had a diagnosis of epilepsy, which appeared to be essentially the only nonpsychiatric use in this population.
As shown in Table 1, the mean peak baseline and follow-up ALT values were higher in each of the groups that had positive hepatitis C status than in the groups with negative hepatitis C status and unknown status. However, marked elevations in ALT were uncommon, even among individuals known to have positive hepatitis C status. For the valproic acid patients, 22.8% of those with positive results for hepatitis C had marked or moderate peak elevations, compared to 9.5% for the group with negative status and 3.2% for those with unknown hepatitis C status, a difference that was significant. Among the patients in the positive hepatitis C status comparison groups, 14.3% of those taking antidepressants and 13.0% of those in the lithium/gabapentin group also had moderate or marked increases in peak ALT level, rates that were not statistically different from that for the patients taking valproic acid who had positive hepatitis C status.
Marked elevation in the peak ALT level was uncommon in the valproic acid groups with negative and unknown status (0.5% and 0.4%, respectively) but was much more frequent in those with positive results for hepatitis C (7.9%). However, 5.6% of the antidepressant comparison group and 6.5% of the lithium/gabapentin group also had marked ALT elevations. The difference among the groups with positive hepatitis C status was not significant. For the patients taking valproic acid who had positive hepatitis C status and had marked ALT elevations, the individual peak ALT values after the beginning of valproic acid treatment ranged from 175 to 439 U/liter.
In order to evaluate possible ALT differences due to differences in doses of valproic acid, the mean serum levels of valproic acid were compared in the groups with positive, negative, and unknown hepatitis C status. The levels ranged between 57.7 and 60.5 U/liter, and there were no significant differences between the three groups.
Discussion
Although hepatitis C status does affect the likelihood that patients will have meaningful increases in ALT levels after starting to take valproic acid, the individuals in this study who had the hepatitis C virus and who were beginning treatment with antidepressants or with lithium or gabapentin had rates of marked ALT elevations similar to those observed in the patients beginning valproic acid treatment who had positive hepatitis C status. These findings suggest that many of the elevations in ALT in the latter group may be due to fluctuations in the hepatitis C disease or other factors (e.g., unacknowledged alcohol use), rather than the valproic acid itself.
After acute infection with the hepatitis C virus, the ALT level typically rises but remains less than 800 IU/liter, although it rarely does exceed 2000 IU/liter (16). During the course of chronic hepatitis C infection, the ALT value varies. In one study (17) the range of ALT values was 4 to 556 IU/liter, and 31% of the subjects had persistently normal levels, 42% had peaks of no more than two times normal, 15% had peaks greater than two times normal, and 12% had peaks greater than three times normal. For our subjects with positive hepatitis C status who had marked ALT elevations, the maximum levels were between 175 and 439 IU/liter and are consistent with elevations commonly seen during the course of chronic hepatitis C infection (16, 17). Whether these ALT elevations are associated with additional histologic damage is currently not known, as no reliable relationship between ALT elevation and degree of histological damage has been found (10, 11).
This study has several limitations. Because the blood samples for ALT measurement were not drawn in association with liver biopsy, and the follow-up was limited to 90 days, we are unable to make statements about the long-term safety of valproic acid for patients with hepatitis C. This was a review of laboratory and pharmacy data only, and not a full chart review, so the ALT elevations may have been due to undetected causes other than medications (e.g., alcohol use). Since the hepatitis C infection was predominantly diagnosed by enzyme immunoassay testing alone and not uniformly confirmed with recombinant immunoblot assay and/or PCR, some patients may have been falsely identified as having the hepatitis C virus. Our group with negative hepatitis C status had higher baseline ALT levels than might be expected in a group of patients without hepatic disease. We speculate that a number of the patients with negative status were tested because they had abnormalities suggesting the presence of liver disease. Therefore, this group of patients may have had more hepatic disease than would be expected in a normal population.
In conclusion, the present findings indicate that valproic acid can be used for many patients who have the hepatitis C virus without adversely affecting ALT levels. However, eight of the 10 patients treated with valproic acid who had marked ALT elevations were in the group with positive hepatitis C status, and valproic acid was associated with increases in ALT in some patients who did not have the hepatitis C virus. So it is important to monitor ALT closely in all patients treated with valproic acid. Because hepatitis C can cause fluctuations in ALT levels, ALT elevations in patients with the hepatitis C virus may not always be due to valproic acid, and it is difficult to know what small ALT increases mean in these patients. Even so, it seems prudent to stop valproic acid treatment for hepatitis C patients whose ALT levels are clearly increased above the normal pretreatment baseline and to consult with a hepatitis specialist. If valproic acid provides the most effective control of psychiatric symptoms for a patient and ALT elevations occur, close collaboration with a hepatitis specialist would be essential. In such cases, providers need to weigh the risk of not adequately treating psychiatric problems against any potential hepatotoxicity of continued treatment with valproic acid.
 |
Received Dec. 18, 2001; revision received Aug. 6, 2002; accepted Aug. 27, 2002. From the Department of Veterans Affairs Puget Sound Health Care System and Northwest Hepatitis C Resource Center. Address reprint requests to Dr. Felker, Department of Veteran Affairs Puget Sound Health Care System, MHC/116, 1660 South Columbian Way, Seattle, WA 98108-1597; [email protected] (e-mail). The conclusions of this article represent those of the authors and do not necessarily represent the opinions of the Department of Veterans Affairs.
1. Davis LL, Ryan W, Adinoff B, Petty F: Comprehensive review of the psychiatric uses of valproate. J Clin Psychopharmacol 2000; 20(suppl 1):1S-17SGoogle Scholar
2. McElroy SL, Keck PE Jr, Pope HG Jr, Hudson JI: Valproate in psychiatric disorders: literature review and clinical guidelines. J Clin Psychiatry 1989; 50(March suppl):23-29Google Scholar
3. Harden CL: Therapeutic safety monitoring: what to look for and when to look for it. Epilepsia 2000; 41(suppl 8):S37-S44Google Scholar
4. Levin TL, Berdon WE, Seigle RR, Nash MA: Valproic-acid-associated pancreatitis and hepatic toxicity in children with endstage renal disease (letter). Pediatr Radiol 1997; 27:192-193Crossref, Medline, Google Scholar
5. Njolstad PR, Skjeldal OH, Afsteribbe E, Huckriede A, Wannag E, Sovik O, Waaler PE: Medium chain acyl-CoA dehydrogenase deficiency and fatal valproate toxicity. Pediatr Neurol 1997; 16:160-162Crossref, Medline, Google Scholar
6. Rimmer EM, Richens A: An update on sodium valproate. Pharmacotherapy 1985; 5:171-184Crossref, Medline, Google Scholar
7. Dreifuss FE, Langer DH, Moline KA, Maxwell JE: Valproic acid hepatic fatalities, II: US experience since 1984. Neurology 1989; 39:201-207Crossref, Medline, Google Scholar
8. Physicians’ Desk Reference, 55th ed. Montvale, NJ, Medical Economics, 2001, p 433Google Scholar
9. Alter MJ, Kruszon-Moran D, Nainan O, McQuillan GM, Gao F, Moyer LA, Kaslow RA, Margolis HS: Risk factors for acute non-A, non-B hepatitis in the United States, 1988 through 1994. N Engl J Med 1999; 341:556-562Crossref, Medline, Google Scholar
10. McCormick SE, Goodman ZD, Maydonovitch CL, Sjogren MH: Evaluation of liver histology, ALT elevation, and HCV RNA titer in patients with chronic hepatitis C. Am J Gastroenterol 1996; 91:1516-1522Medline, Google Scholar
11. Haber MM, West AB, Haber AD, Reuben A: Relationship of aminotransferases to liver histological status in chronic hepatitis C. Am J Gastroenterol 1995; 90:1250-1257Medline, Google Scholar
12. Schmidt E, Schmidt FW: Progress in the enzyme diagnosis of liver disease: reality or illusion? Clin Biochem 1990; 23:375-382Crossref, Medline, Google Scholar
13. Zdilar D, Franco-Bronson K, Buchler N, Locala JA, Younossi ZM: Hepatitis C, interferon alfa, and depression. Hepatology 2000; 31:1207-1211Crossref, Medline, Google Scholar
14. Rosenberg SD, Goodman LA, Osher FC, Swartz MS, Essock SM, Butterfield MI, Constantine NT, Wolford GL, Salyers MP: Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health 2001; 91:31-37Crossref, Medline, Google Scholar
15. Saxon AJ, Sloan KL, Reoux J, Haver VL: Safety of disulfiram for patients with abnormal liver function tests. J Clin Psychiatry 1998; 59:313-316Crossref, Medline, Google Scholar
16. Larson AM, Carithers RL: Hepatitis C in clinical practice. J Intern Med 2001; 249:111-120Crossref, Medline, Google Scholar
17. Conry-Cantilena C, VanRaden M, Gibble J, Melpolder J, Shakil AO, Viladomiu L, Cheung L, DiBisceglie A, Hoofnagle J, Shih JW, Kaslow R, Ness P, Alter HJ: Routes of infection, viremia, and liver disease in blood donors found to have hepatitis C virus infection. N Engl J Med 1996; 334:1691-1696Crossref, Medline, Google Scholar



