Are Depressive Symptoms a Risk Factor for Mortality in Elderly Japanese American Men?: The Honolulu-Asia Aging Study
Abstract
OBJECTIVE: This study determined the influence of depressive symptoms on subsequent mortality of all causes. METHOD: The Honolulu Heart Program, established in 1965, is a prospective, community-based cohort of Japanese American men living in Hawaii. The analysis was based on 3,196 Japanese American men aged 71–93 at the time of the fourth examination in 1991–1993. Depressive symptoms were measured by use of an 11-question version of the Centers for Epidemiologic Studies Depression Scale questionnaire. All-cause mortality data were available for 6 years of follow up. Data were analyzed on the basis of presence or absence of chronic diseases. RESULTS: The overall prevalence of frequent depressive symptoms was 9.9%. Age-adjusted mortality rates at 3 years were 48.0 and 30.3 per 1,000 person-years for the depressed and nondepressed groups, respectively. At 6 years, the rates were 54.1 (depressed) and 41.5 (nondepressed) per 1,000 person-years. After adjustment for age, marital status, and antidepressant use, the relative risk for all-cause mortality associated with depressive symptoms was 1.53 for 3-year and 1.27 for 6-year mortality. Among participants who were healthy (without cognitive impairment, coronary heart disease, stroke, diabetes, or cancer), the association between depressive symptoms and mortality was greater (relative risk of 2.30 and 1.57 for 3- and 6-year mortality, respectively). Among participants with chronic disease, there were no significant associations between depressive symptoms and mortality. CONCLUSIONS: Depressive symptoms are a risk factor for mortality in elderly people, particularly in physically healthy individuals.
Depressive symptoms are common in community-dwelling elderly people (1). Yet the diagnosis of depression has often been missed and treatment inadequate (2). The result of ignoring depression has serious implications. Depression amplified the morbidity of disability, pain, drug side effects, and malnutrition and increased the need for health care in one study (3). Mortality was higher in community studies of depressed elderly persons (4–10) and especially in depressed elderly persons with coronary heart disease (11–14). A recent community study (15) noted a nearly twofold greater cardiac mortality for individuals with major depression than those with minor depression.
We present data on the contribution of depressive symptoms to mortality in elderly Japanese American men. We hypothesized that depressive symptoms would result in greater mortality and that these rates would be particularly high in the physically ill.
Method
Study Population
The Honolulu Heart Program began as a prospective cardiovascular study of 8,006 men of Japanese ancestry living on the island of Oahu, Hawaii, in 1965 who were born between 1900 and 1919. All men of Japanese ancestry identified by using World War II selective service registration cards were invited to participate (16). Since 1965 the cohort has been examined seven times. More recent examinations have focused on neurologic diseases and conditions associated with aging.
This analysis was based on the fourth examination of the cohort, conducted from 1991 to 1993, when 3,741 men aged 71–93 years were examined (80% of the 4,676 eligible cohort). All-cause mortality data were available for 6 years of follow-up. The study was approved by the institutional review board of Kuakini Medical Center, the procedures followed were in accordance with institutional guidelines, and after complete description of the study to the subjects, written informed consent was obtained.
Data Collection
The fourth examination included collection of demographic information, answers to medical and psychological questionnaires, assessment of cognitive function, fasting blood tests, a 2-hour glucose tolerance test, seated blood pressure and anthropometry measures that were collected in a standardized manner. Morbidity and mortality surveillance included monitoring of hospital discharge records, death records, and death certificates. For this report, all-cause mortality data were analyzed and matched with the national death index through December 1997. Mortality data collection was believed to be essentially complete; at the fourth examination, only five men were lost to follow-up.
Depressive Symptoms
Participants were screened for depressive symptoms by using an 11-question version of the Centers for Epidemiologic Studies Depression (CES-D) Scale questionnaire (Appendix 1). Participants who did not answer three or more of the 11 depression questions were excluded from this analysis, leaving 3,196 participants to be studied. The standard CES-D Scale uses a cutoff score of 16 points for depressive symptoms (17). In this 11-question version, a score of 9 or greater was used (determined by extrapolation; 16/20 × 11=8.8, rounded up to 9). Shortened forms of the CES-D Scale have been found to be comparable with the full-scale version (18, 19). For convenience, we will refer to presence of depressive symptoms as “depression” or “depressed”; the term is not synonymous with clinical depression.
Other Key Variables
Covariates were selected because of their potential relationship with depressive symptoms or mortality. Diabetes mellitus was defined by World Health Organization criteria, by history (as told by the subject to a physician), by taking medications (insulin or oral hypoglycemics), by a fasting glucose level of 140 mg/dl or more, or by a 2-hour postload glucose of 200 mg/dl or more (20). Body mass index was defined as weight in kilograms divided by height in meters squared. The Physical Activity Index was based on the one used in the Framingham Study (21) and the Honolulu Heart Program (22), which multiplied the oxygen consumption of five different levels of activity with the numbers of hours a day engaged in that task.
Antidepressant medication use was determined by observation; participants brought in medications used in the previous 2 weeks. Cognitive function was measured with the Cognitive Abilities Screening Instrument (23), which was developed for cross-cultural studies of dementia. A score on the Cognitive Abilities Screening Instrument of less than 74 defined cognitive impairment. We tested the accuracy of a consensus panel of three physicians using DSM-III-R criteria for determination of dementia after a neurologist’s examination and neuropsychological testing of 426 participants; when we used the cutoff point of 74, the sensitivity of the instrument for dementia was 80%, and the specificity was 90%.
Statistical Analysis
Subjects were divided into two groups on the basis of presence or absence of depressive symptoms, as defined. Means of variables were compared in these two groups by using two-sample t tests for continuous variables and chi-square tests for categorical variables. Age-adjusted mortality rates were calculated for 3-year and 6-year mortality per 1,000 person-years of follow-up. Dose-response relationships of depressive symptoms with mortality were studied for the six groups on the basis of the 11-item CES-D Scale score. Kaplan-Meier survival curves were plotted for 6-year mortality by using Wilcoxon’s test for statistical significance.
Three separate Cox proportional hazards models assessed the association of depressive symptoms and mortality. Separate models for 3-year and 6-year mortality analyzed the immediate and long-term effects of depressive symptoms. The first model adjusted for age, marital status, and antidepressants. The second model added cardiovascular risk factors (body mass index, score on Physical Activity Index, systolic blood pressure, and presence of smoking and diabetes mellitus). The third model added chronic diseases (cognitive impairment, coronary heart disease, stroke, and cancer).
To study the effect of illness on the association between depression and mortality, we created two groups: “physically ill” (cognitive impairment, coronary heart disease, stroke, diabetes, or cancer) and “healthy” (individuals without chronic diseases). We repeated the Cox proportional hazards models for those with and without chronic diseases. In this cohort, coronary heart disease, stroke, and cancer were known to be the three most frequent causes of death (24). All statistical analyses were performed using SAS software (SAS Institute, Cary, N.C.). All of the statistical tests were two-tailed.
Results
A total of 317 participants (9.9% of the cohort) had a score of 9 or greater on the 11-item CES-D Scale and were considered “depressed” (mean=3.73, SD=3.68, median=3.0, mode=3.0, range=0–26). The prevalence of depressive symptoms showed little variation among age groups (ages 71–74, 8.83%; ages 75–79, 10.5%; ages 80–84, 10.47%; ages 85 and older, 9.96%) (χ2=2.05, df=3, p=0.56).
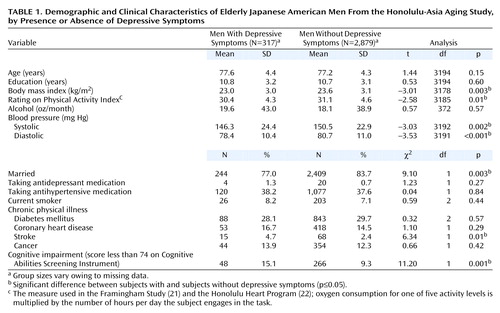
Those with depressive symptoms were significantly less likely to be married, had a lower body mass index, a lower rating on the Physical Activity Index, and lower systolic and diastolic blood pressure. They were more likely to have cognitive impairment and stroke (Table 1). The associations with age, education, smoking, use of antidepressants or antihypertensives, alcohol, diabetes, coronary heart disease, and cancer were not significant.
The follow-up interval was from the initial measure of depressive symptoms in 1991–1993 until December 1997. During this interval, there were 684 deaths in the cohort: 601 deaths among the nondepressed and 83 among the depressed (Table 2). Kaplan-Meier survival curves showed significantly greater mortality for men with depressive symptoms (Figure 1).
Three separate Cox proportional hazards models were analyzed with all-cause mortality as the endpoint (Table 3). In the first model, after adjustment for age, marital status, and antidepressant medication use, the relative risk for all-cause mortality associated with depressive symptoms was 1.53 (Cox proportional hazards model, 95% confidence interval [CI]=1.09–2.16; χ2=5.88, df=1, p=0.02) for 3-year mortality and 1.27 (Cox proportional hazards model, 95% CI=1.01–1.60, χ2=4.26, df=1, p=0.04) for 6-year mortality. Adjustment for other factors known to influence mortality (model 2) did not appreciably alter the relative risk for either 3- or 6-year mortality. However, in model 3, the association no longer reached statistical significance after further adjustment for presence of chronic diseases.
To avoid the influence of these diseases on depressive symptoms and mortality, Cox proportional hazards models were repeated separately for elderly men with and without chronic diseases (Table 3>). As defined previously, the healthy group excluded participants with cognitive impairment, coronary heart disease, stroke, diabetes, or cancer. The physically ill group included participants with at least one of these diseases. Associations between depression and mortality were significant only in the healthy group. The associations were stronger for 3-year than for 6-year mortality. We found a significant interaction among health/physical illness, presence of depressive symptoms, and their association with 3-year mortality but no significant interaction for 6-year mortality (data not shown).
Discussion
Depressive symptoms predicted greater mortality in elderly Japanese American men, a risk that continued after adjustment for age and chronic medical conditions. To our knowledge, this is the first community-based study of an Asian population in terms of depression and mortality. An extensive review showed that previous studies were predominantly among Caucasian populations (4). We found only one article that analyzed this association in Mexican Americans (5).
Most community studies showed greater mortality in depressed patients (4–6, 8–10), although there was no association between depressive symptoms and mortality in the Piedmont Health Survey (7). A literature review (4) noted an average relative risk of 1.7 (range=1.6–1.8). Others reported excess mortality for all psychiatric disorders (25) and a relative risk of 1.5–2.5 in elderly persons with organic mental disorders, mood disorders, and psychotic disorders (26).
The most unexpected finding was that the physically healthy, depressed elderly men had a stronger association with mortality compared with the physically ill group—a result opposite to that of previous reports (4–14). We believe we are the first investigators to divide the patient cohort into healthy and physically ill groups, thus building in a comparison group. Others have either used physically healthy groups alone, controlling for medical burden, or analyzed a pure physically ill cohort (4–14).
We wondered about possible explanations. Perhaps depression was a marker for undiagnosed comorbid medical illness. The additive effect of depression on mortality may be minimal compared with the burden of chronic disease. Differences in the genetic composition and psychosocial environment of Japanese Americans in Hawaii could have major differences on the course of depression. As another example, strong associations between depression and cardiovascular illness (11–15), cerebrovascular disease (27), and diabetes (28) have been reported. Our study showed a relationship of depression with only cerebrovascular disease.
Limitations of the Study
This study analyzed only male elderly patients of Japanese ancestry in a community population. Although we controlled for the effect of gender and ethnicity, these findings may not be generalizable to other groups or settings. Compared with other study groups, our cohort was essentially homogeneous in terms of gender, age, and ethnicity. Furthermore, most of the Japanese population in Hawaii arrived from rural southern Japan at the turn of the century (29), so there may be similarities, even at the genetic level.
We were concerned that 545 participants were excluded from the study because of incomplete or invalid answers on the 11-item CES-D Scale questionnaire. We postulated that such individuals were unable to answer questions because of more severe cognitive impairment and/or medical burden. In addition, there were 935 survivors (20%) in the cohort that did not participate in examination 4; this group was most likely less healthy than the participants. Indeed, we found that mortality rates were more than double in those who participated in examination 4 but did not complete a valid 11-item CES-D Scale questionnaire and more than triple in those who did not participate at all in examination 4 (data not shown). These two excluded groups probably had the most severe medical burden and perhaps a high rate of depression. The elimination of these groups from the analysis could have skewed our evaluation of depression in the physically ill cohort.
Perhaps the most significant limitation of this study was that depression was measured at one point in time and followed prospectively. Depression-related mortality may have occurred before the measurement of depressive symptoms. Unfortunately, data on depressive symptoms were not collected at earlier time points. Nonetheless, the deleterious effect of depression on mortality may not occur until middle age. Vaillant (30) showed no differences in health in a cohort of college-educated men until age 50. By age 70, there were significant differences in the mortality of healthy versus depressed men.
Conclusions
In summary, our study confirmed the hypothesis that depressive symptoms result in greater mortality for Japanese American elderly men, a group not previously studied, to our knowledge. Compared with other studies, our cohort was particularly homogenous. We felt that dividing the cohort into “healthy” and “physically ill” groups represented a novel way of studying the relationship among depression, health, and medical burden. Other databases could be analyzed in a similar manner to see if our findings could be replicated.
 |
 |
 |
Presented in part at the 151st annual meeting of the American Psychiatric Association, Toronto, May 30 to June 4, 1998; the 56th annual meeting of the American Geriatric Society, Philadelphia, May 20–23, 1999; and the 14th annual meeting of the American Association for Geriatric Psychiatry, San Francisco, Feb. 23–26, 2001. Received Dec. 20, 2000; revisions received May 25, 2001, and Jan. 2, 2002; accepted Feb. 18, 2002. From the Honolulu-Asia Aging Study, Kuakini Medical Center, Honolulu; the Geriatric Medicine Program and the Department of Psychiatry, John A. Burns School of Medicine, Honolulu; and the Epidemiology, Demography, and Biometry Program, National Institute on Aging, NIH, Bethesda, Md. Address reprint requests to Dr. Takeshita, 1356 Lusitana St., 4th Floor, Honolulu, Hawaii 96813; [email protected] (e-mail). Supported by NIH contract AG-42149 from the National Institute on Aging and HC-05102 from the National Heart, Lung, and Blood Institute.
 |
APPENDIX 1.
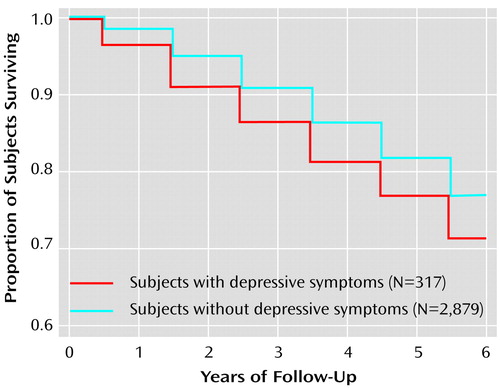
Figure 1. Probability of Mortality in Elderly Japanese American Men From the Honolulu-Asia Aging Study, by Depressive Symptomsa
aKaplan-Meier survival curves for 6-year mortality from all causes in relation to presence or absence of depressive symptoms at examination 4 (Wilcoxon’s χ2=6.71, df=1, p=0.01).
1. Blazer D, Hughes DC, George LK: The epidemiology of depression in an elderly community population. Gerontologist 1987; 27:281-287Crossref, Medline, Google Scholar
2. Reynolds CF, Kupfer DJ: Depression and aging: a look to the future. Psychiatr Serv 1999; 50:1167-1172Link, Google Scholar
3. Katz IR: On the inseparability of mental and physical health in aged persons: lessons from depression and medical comorbidity. Am J Geriatr Psychiatry 1996; 4:1-16Google Scholar
4. Wulsin LR, Vaillant GE, Wells VE: A systematic review of the mortality of depression. Psychosom Med 1999; 61:6-17Crossref, Medline, Google Scholar
5. Black SA, Markides KS: Depressive symptoms and mortality in older Mexican Americans. Ann Epidemiol 1999; 9:45-52Crossref, Medline, Google Scholar
6. Murphy JM, Monson RR, Olivier DC, Sobol AM, Leighton AH: Affective disorders and mortality. Arch Gen Psychiatry 1987; 44:473-480Crossref, Medline, Google Scholar
7. Fredman L, Schoenbach VJ, Kaplan BH, Blazer DG, James SA, Kleinbaum DG, Yankaskas B: The association between depressive symptoms and mortality among older participants in the Epidemiologic Catchment Area—Piedmont Health Survey. J Gerontol 1989; 44:S149-S156Google Scholar
8. Bruce ML, Leaf PJ, Rozal GP, Florio L, Hoff RA: Psychiatric status and 9-year mortality data in the New Haven Epidemiologic Catchment Area study. Am J Psychiatry 1994; 151:716-721Link, Google Scholar
9. Cole MG, Bellavance F, Mansour A: Prognosis of depression in elderly community and primary care populations: a systematic review and meta-analysis. Am J Psychiatry 1999; 156:1182-1189Abstract, Google Scholar
10. Penninx BWJH, Geerlings SW, Deeg DJH, van Eijk JTM, van Tilburg W, Beekman ATF: Minor and major depression and the risk of death in older persons. Arch Gen Psychiatry 1999; 56:889-895Crossref, Medline, Google Scholar
11. Carney RM, Freedland KE, Veith RC, Jaffe AS: Can treating depression reduce mortality after an acute myocardial infarction? Psychosom Med 1999; 61:666-675Crossref, Medline, Google Scholar
12. Lespérance F, Frasure-Smith N, Talajic M: Major depression before and after myocardial infarction: its nature and consequences. Psychosom Med 1996; 58:99-110Crossref, Medline, Google Scholar
13. Simonsick EM, Wallace RB, Blazer DG, Berkman LF: Depressive symptomatology and hypertension-associated morbidity and mortality in older adults. Psychosom Med 1995; 57:427-435Crossref, Medline, Google Scholar
14. Koenig HG, George LK, Larson DB, McCullough ME, Branch PS, Kuchibhatla M: Depressive symptoms and nine-year survival of 1,001 male veterans hospitalized with medical illness. Am J Geriatr Psychiatry 1999; 7:124-131Crossref, Medline, Google Scholar
15. Penninx BWJH, Beekman ATF, Honig A, Deeg DJH, Schoevers RA, van Eijk JTM, van Tilburg W: Depression and cardiac mortality: results from a community-based longitudinal study. Arch Gen Psychiatry 2001; 58:221-227Crossref, Medline, Google Scholar
16. Worth RM, Kagan A: Ascertainment of men of Japanese ancestry in Hawaii through World War II selective service registration. J Chronic Dis 1970; 23:389-397Crossref, Medline, Google Scholar
17. Radloff LS, Teri L: Use of the Center for Epidemiological Studies Depression Scale with older adults, in Clinical Gerontology: A Guide to Assessment and Intervention. Edited by Brink TL. New York, Haworth Press, 1986, pp 119-135Google Scholar
18. Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley JC: Two shorter forms of the CES-D depression symptoms index. J Aging Health 1993; 5:179-192Crossref, Medline, Google Scholar
19. Turvey CL, Wallace RB, Herzog R: A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. Int Psychogeriatr 1999; 11:139-148Crossref, Medline, Google Scholar
20. Rodriguez BL, Curb JD, Burchfiel CM, Huang B, Sharp DS, Lu GY, Fujimoto W, Yano K: Impaired glucose tolerance, diabetes, and cardiovascular disease risk factor profiles in the elderly. Diabetes Care 1996; 19:587-590Crossref, Medline, Google Scholar
21. Kannel WB, Sorlie P: Some health benefits of physical activity: the Framingham Study. Arch Intern Med 1979; 139:857-861Crossref, Medline, Google Scholar
22. Burchfiel CM, Sharp DS, Curb JD, Rodriguez BL, Hwang LJ, Marcus EB, Yano K: Physical activity and incidence of diabetes: the Honolulu Heart Program. Am J Epidemiol 1995; 141:360-368Crossref, Medline, Google Scholar
23. Teng EL, Hasegawa K, Homma A, Imai Y, Larson E, Graves A, Sugimoto K, Yamaguchi T, Sasaki H, Chiu D, White LR: The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr 1994; 6:45-58Crossref, Medline, Google Scholar
24. Curb JD: Mortality, in The Honolulu Heart Program: An Epidemiological Study of Coronary Heart Disease and Stroke. Edited by Kagan A. Amsterdam, Harwood Academic, 1996, pp 127-134Google Scholar
25. Harris EC, Barraclough B: Excess mortality of mental disorder. Br J Psychiatry 1998; 173:11-53Crossref, Medline, Google Scholar
26. Zubenko GS, Mulsant BH, Sweet RA, Pasternak RE, Tu XM: Mortality of elderly patients with psychiatric disorders. Am J Psychiatry 1997; 154:1360-1368Link, Google Scholar
27. Jonas BS, Mussolino ME: Symptoms of depression as a prospective risk factor for stroke. Psychosom Med 2000; 62:463-471Crossref, Medline, Google Scholar
28. Talbot F, Nouwen A: A review of the relationship between depression and diabetes in adults: is there a link? Diabetes Care 2000; 23:1556-1562Crossref, Medline, Google Scholar
29. DeFrancis J: Things Japanese in Hawaii. Honolulu, University Press of Hawaii, 1973Google Scholar
30. Vaillant GE: Natural history of male psychological health, XIV: relationship of mood disorder vulnerability to physical health. Am J Psychiatry 1998; 155:184-191Abstract, Google Scholar



