Historical, Psychopathological, Neurological, and Neuropsychological Aspects of Deficit Schizophrenia: A Multicenter Study
Abstract
OBJECTIVE: This multicenter study aimed to verify whether the historical and psychopathological characteristics of a large group of patients with deficit schizophrenia were consistent with those reported in previous studies. The authors also tested the hypothesis that neurological and neuropsychological indices sensitive to frontoparietal dysfunction, but not those sensitive to temporal lobe dysfunction, are more impaired in patients with deficit schizophrenia than in those with nondeficit schizophrenia. METHOD: For each patient with deficit schizophrenia enrolled in the study, a matched subject with nondeficit schizophrenia was recruited. Historical, psychopathological, neurological, and neuropsychological evaluations were carried out for all patients. RESULTS: Patients with deficit schizophrenia, compared with patients with nondeficit schizophrenia, had a similar severity of positive symptoms and disorganization and less hostility. They had poorer premorbid adjustment during childhood and early adolescence and exhibited more impairment in general cognitive abilities. The deficit state was associated with impairment in sequencing of complex motor acts, which suggests frontoparietal dysfunction. CONCLUSIONS: Previous reports of differences in historical, psychopathological, and neuropsychological characteristics between patients with deficit schizophrenia and those with nondeficit schizophrenia were mostly supported by the present findings. Neurological findings suggest that frontoparietal functioning is more impaired in patients with deficit schizophrenia. Deficit schizophrenia might represent a neurodevelopmental subtype of schizophrenia in which significant behavioral and cognitive impairment since childhood compromises the development of basic capacities relevant to subsequent cognitive and social functioning.
Research on schizophrenia has been seriously hindered by the extreme interindividual variability of the clinical manifestations of the disorder, which probably reflects a multiplicity of etiopathogenetic mechanisms (1–3). The concept of deficit schizophrenia was introduced to identify a relatively homogeneous subgroup of patients characterized by the presence of enduring primary negative symptoms: restricted affect, diminished emotional range, poverty of speech, curbing of interests, lack of a sense of purpose, and diminished social drive (4).
The prevalence of deficit schizophrenia has been reported to be about 15% among patients with first-episode schizophrenia and 25%–30% among those with chronic schizophrenia (5). Longitudinal studies have demonstrated that the deficit/nondeficit categorization is highly stable (6). Patients with deficit schizophrenia, relative to those with the nondeficit subtype, have been found to have poorer premorbid adjustment (7–9), a greater genetic load for schizophrenia (5, 10–12), a more frequently insidious onset of illness (7), a similar severity of productive symptoms and a lower severity of dysphoria (5, 13), a more frequent resistance to antipsychotic treatment (14), and a worse long-term outcome (7).
Neuropsychological studies have reported that patients with deficit schizophrenia are significantly more impaired than those with nondeficit schizophrenia on measures sensitive to frontal and parietal lobe dysfunction but not on those sensitive to temporal lobe dysfunction (15–17). Investigations that have included a structured neurological examination have shown a greater impairment in sensory integration—but not in motor coordination or sequencing of complex motor acts—among subjects with deficit schizophrenia (8, 18). Functional brain imaging investigations have suggested cortical-striatal-thalamocortical circuit dysfunction, including prefrontal and parietal regions, as the neural basis for deficit symptoms (19–21).
Here we report the results of a large multicenter study that aimed to verify whether the historical and psychopathological characteristics of a large group of patients with deficit schizophrenia were consistent with the pattern that has emerged from previous studies. In addition, this study sought to test the hypothesis that neurological and neuropsychological indices sensitive to frontoparietal dysfunction (attention and visuospatial abilities), but not those sensitive to frontotemporal dysfunction (verbal and visual explicit memory), are more impaired in patients with deficit schizophrenia than in those with nondeficit schizophrenia.
Method
Patient Group and Recruitment Procedures
The study was carried out with patients who were regularly attending the outpatient units, day-care programs, and rehabilitation centers of the University Psychiatric Departments of Naples, Milan, L’Aquila, and Pisa, Italy. Those who had a clinical diagnosis of schizophrenia and were reported to be clinically stable were tested to verify that they met the following inclusion criteria: 1) a DSM-IV diagnosis of schizophrenia, confirmed by the Structured Clinical Interview for DSM-IV (SCID); 2) age between 16 and 55 years; 3) no history of severe mental retardation, alcoholism, or drug abuse or dependence in the last 12 months and no previous ECT; 4) no significant changes in the clinical state or in drug treatment during the preceding 3 months; and 5) willingness to participate in the study procedures, expressed by providing written informed consent after complete description of the study. Patients meeting these criteria were then classified as having either deficit or nondeficit schizophrenia after being interviewed with the Schedule for the Deficit Syndrome (22).
The original version of the Schedule for the Deficit Syndrome was translated into Italian, back-translated into English by a bilingual translator, and discussed with one of the authors of the schedule (Brian Kirkpatrick), who also guided the training of the investigators. At the end of the training, there was no disagreement among eight raters (Cohen’s kappa=1) in classifying one patient with nondeficit schizophrenia and two patients with deficit schizophrenia. Intraclass correlation coefficients (ICCs) showed good agreement among raters for the Schedule for the Deficit Syndrome measures of restricted affect (0.76), diminished emotional range (0.62), poverty of speech (0.67), curbing of interests (0.74), diminished sense of purpose (0.74), and diminished social drive (0.81). Investigators were also trained in the use of the SCID and the SCID Nonpatient Version. At the end of the training, there was no disagreement among eight raters (Cohen’s kappa=1) in classifying two patients with schizophrenia and one as having bipolar I disorder with mood-incongruent psychotic features. The same kappa value was achieved by six raters on the nonpatient SCID.
All subjects with deficit schizophrenia according to the Schedule for the Deficit Syndrome were enrolled in the study. For each recruited patient with deficit schizophrenia, an age- and sex-matched patient with nondeficit schizophrenia was recruited. Thus, 60 deficit/nondeficit pairs were recruited. However, only those who completed most of the study procedures were included in the data analysis. The final group consisted of 58 patients with deficit schizophrenia (43 men and 15 women) and 54 patients with nondeficit schizophrenia (41 men and 13 women). The comparability between the two groups with respect to demographic and clinical variables was assessed by analyses of variance (ANOVAs) or chi-square tests, as appropriate. There were no significant differences between the deficit and nondeficit patients in terms of age (mean=35.2 years [SD=7.3] and 34.4 years [SD=7.7], respectively), education (mean=11.4 years [SD=3.1] and 11.3 years [SD=3.3]), age at onset of illness (mean=21.6 years [SD=4.5] and 21.7 years [SD=4.2]), duration of illness (mean=13.6 years [SD=7.4] and 12.8 years [SD=6.9]), or gender distribution. The mean current antipsychotic dose was significantly higher in patients with nondeficit schizophrenia (mean=645 mg/day [SD=410] in chlorpromazine equivalents) than in patients with deficit schizophrenia (mean=445 mg/day [SD=363]) (F=7.23, df=1, 106, p<0.008). There was no difference between the deficit and nondeficit patient groups as to the type of antipsychotic treatment they were receiving (novel antipsychotics: 56.9% [N=33] and 46.3% [N=25], respectively; standard neuroleptics: 25.9% [N=15] and 27.8% [N=15]; combination: 13.8% [N=8] and 24.1% [N=13]).
In two of the participating centers (Naples and Milan), a group of 26 healthy subjects (18 men and eight women)—matched to patients for age (within 3 years), education (within 3 years), handedness, and sex—was recruited through flyers from the general population for comparisons on neuropsychological indices. These comparison subjects had no personal or family history of major psychiatric disorders—as ascertained by the nonpatient SCID and the Family History Questionnaire and Relative Psychiatric History Questionnaires (23)—and no history of severe head trauma or substance-related disorders. Their mean age of 34.6 years (SD=9.4) and their mean education level of 12.7 years (SD=2.9) did not significantly differ from those of patients.
Study Procedures
Different investigators carried out historical, psychopathological, neurological, and neuropsychological evaluations to avoid “halo” effects. All of them, as well as patients and family members, were blind to the deficit/nondeficit categorization and had not been informed of the specific study aims and hypotheses.
The personal and family history of each recruited patient was explored with the help of at least one family member (the mother, if available) by using the Family History Questionnaire and Relative Psychiatric History Questionnaires, the Obstetric Complications Scale (24), and the Premorbid Adjustment Scale (25). The psychopathological state was evaluated by the expanded Brief Psychiatric Rating Scale (BPRS), version 4.0 (26), the Scale for the Assessment of Negative Symptoms (SANS) (27), and the Scale for the Assessment of Positive Symptoms (SAPS) (28). The recent history of hospitalization and social functioning was explored by the Strauss-Carpenter Scale (29). Investigators were trained in the use of these instruments, and at the end of the training, good to excellent agreement was observed among 12 raters who assessed three patients (for the expanded BPRS, ICC=0.61–1.00; for the SANS, ICC= 0.83–0.99 [except for the alogia and attention subscales, which had coefficients of 0.59 and 0.57, respectively]; for the SAPS, ICC=0.63–1.00).
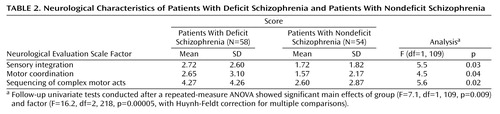
The Neurological Evaluation Scale (30), which assesses three functional areas of interest (sensory integration, motor coordination, and sequencing of complex motor acts) plus some miscellaneous items (abnormalities in eye movements, frontal release signs, and short-term memory), was used for the neurological evaluation. The presence of spontaneous or induced abnormal involuntary movements was assessed by the Abnormal Involuntary Movement Scale (AIMS) (31). Extrapyramidal signs were evaluated by the Simpson-Angus Scale (32). Investigators were trained in the use of these instruments, and at the end of the training, good to excellent agreement was observed among five raters who assessed three patients (for the Neurological Evaluation Scale indices, ICC=0.75–0.99; for the AIMS, ICC=0.95 [total score] and 0.97 [perioral region subtotal]; for the Simpson-Angus Scale average score, ICC=0.54).
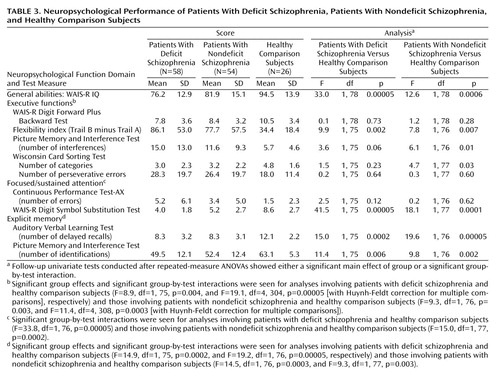
Neuropsychological functioning of patients and healthy comparison subjects was evaluated by their performance on tests that assessed five functional domains: 1) general abilities (WAIS-R IQ results); 2) executive functions (number of categories and perseverative errors on the Wisconsin Card Sorting Test [33], number of interferences on the Picture Memory and Interference Test [34], the part B minus part A time on the Trail Making Test [35], and WAIS-R Digit Forward Plus Backward Test results); 3) focused/sustained attention (WAIS-R Digit Symbol Substitution Test results and the number of errors on the Continuous Performance Test-AX [36]); 4) explicit memory (number of delayed recalls on the Auditory Verbal Learning Test [34] and the number of identifications on the Picture Memory and Interference Test [34]); and 5) visuospatial abilities (Benton Judgment of Line Orientation [37] and the WAIS-R Block Design results).
The Continuous Performance Test-AX was administered by computer; all other tests used the traditional manual method. Tests were administered in the morning (from 9:00 a.m. to 12:00 noon) in a fixed order (Auditory Verbal Learning Test, Benton Judgment of Line Orientation, Wisconsin Card Sorting Test, WAIS-R, Continuous Performance Test, Picture Memory and Interference Test, Trail Making Test [part A], Trail Making Test [part B]).
Data Analyses
Data distributions were examined for normality and homogeneity of variance. In cases in which these assumptions were violated, data were log transformed.
Categorical variables were analyzed by the chi-square test. For continuous variables, in order to deal with the problem of multiple comparisons, the following four strategies were adopted. First, for each investigated area, the lowest possible number of indices was included. Second, ANOVAs were performed, with multivariate or repeated-measures designs used when deemed appropriate. Third, Huynh-Feldt correction for multiple comparisons was used when needed. Fourth, univariate follow-up tests were carried out only when a significant main effect or interaction had been found in the multivariate test.
Psychopathological variables were reduced to eight, on the basis of previous factor analysis studies of the instruments used in this study. Measures from the SANS and SAPS were grouped into three dimensions (38–41): 1) negative symptoms (sum of global scores on the alogia, anhedonia, affective flattening, and avolition subscales of the SANS); 2) reality distortion (sum of global scores on the hallucinations and delusions subscales of the SAPS); and 3) disorganization (sum of global scores on the formal thought disorder and bizarre behavior subscales of the SAPS). Measures from the expanded BPRS were grouped into five factors (42): 1) thought disturbance (sum of scores on the conceptual disorganization, hallucinatory behavior, and unusual thought content items); 2) activation (sum of scores on the tension, mannerisms/posturing, and excitement items); 3) anxiety/depression (sum of scores on the anxiety, guilt feelings, and depressed mood items); 4) hostility/suspiciousness (sum of scores on the suspiciousness, hostility, and uncooperativeness items); and 5) anergia (sum of scores on the blunted affect, emotional withdrawal, and motor retardation items).
For the Neurological Evaluation Scale, the score on each of the three factors was included in the analysis; they were preferred to the total score as they test different functions of the CNS.
Independent two-way ANOVAs for repeated measures on SANS/SAPS dimensions, expanded BPRS factors, and Neurological Evaluation Scale factors were used to test group differences between patients with deficit schizophrenia and patients with nondeficit schizophrenia (group, between; dimension/factor, within). A repeated-measure ANOVA was not deemed appropriate for the Premorbid Adjustment Scale, since the subscores were obtainable only for epochs preceding the age at illness onset, which was different for different subjects, yielding missing data for many of them for some epochs. In this case, a Bonferroni correction was applied (p=0.05/5=0.01).
Neuropsychological variables were grouped into the five aforementioned functional domains. To deal with the problem of multiple comparisons, a multivariate analysis of variance (MANOVA) on all neuropsychological indices was used to assess group differences among subjects with deficit schizophrenia, subjects with nondeficit schizophrenia, and healthy comparison subjects. Only in the presence of a MANOVA significant group effect were follow-up two-way ANOVAs for repeated measures performed on each neuropsychological domain, with diagnosis (deficit schizophrenia, nondeficit schizophrenia, healthy comparison) as a between-group factor and test as a within-group factor. Analyses of covariance (ANCOVAs), with general abilities (the summed age-corrected scores on the WAIS-R vocabulary and picture completion subscales) entered as a covariate, were planned to exclude the influence of group IQ differences on the other tested functions (43). For those domains showing significant group effects or interactions, follow-up analyses of simple effects were planned to locate the source of the main effect or interaction.
For measures showing significant differences between patients with deficit schizophrenia and patients with nondeficit schizophrenia, a further question was addressed, i.e., whether the deficit state remained significantly associated with these measures when combinations of several confounding variables (demographic or clinical) were simultaneously controlled. To this aim, stepwise multiple regression analyses were performed in which the deficit/nondeficit categorization, the SANS/SAPS dimensions, age, education, duration of illness, the Simpson-Angus average score, and the chlorpromazine-equivalent doses were entered as independent variables.
Results
Historical, Psychopathological, and Neurological Evaluations
A family history of schizophrenia was more frequent in patients with deficit schizophrenia (15.5%, N=9) than in those with nondeficit schizophrenia (11.1%, N=6), and a family history of affective disorders was less frequent (10.3% [N=6] versus 13.0% [N=7], respectively), but these differences did not reach statistical significance. The two patient groups were also not significantly different with respect to the frequency of pre- and perinatal complications.
ANOVAs on Premorbid Adjustment Scale scores revealed that patients with deficit schizophrenia, as compared with those with nondeficit schizophrenia, had worse premorbid adjustment during childhood and early and late adolescence; they also had a poorer average premorbid adjustment (Figure 1). Premorbid Adjustment Scale scores for childhood and early adolescence in the nondeficit schizophrenia group were comparable to those reported in the literature for groups of healthy subjects (25).
The two repeated-measure ANOVAs on the expanded BPRS factors and the SANS/SAPS dimensions (Table 1) showed significant group-by-dimension/factor interactions. Follow-up univariate tests showed that patients with deficit schizophrenia had higher scores on the expanded BPRS anergia factor and the SANS negative dimension, and lower scores on the expanded BPRS hostility factor, than did patients with nondeficit schizophrenia (Table 1). For Strauss-Carpenter Scale scores, there was a significant group-by-item interaction (F=5.77, df=3, 327, p<0.01), which was due to fewer social contacts among patients with deficit schizophrenia (mean=1.2, SD=1.4) than among patients with nondeficit schizophrenia (mean=1.8, SD=1.5) (F=4.60, df=1, 109, p=0.03).
On the Neurological Evaluation Scale, a repeated-measure ANOVA showed significant main effects of group and factor; follow-up univariate tests showed that patients with deficit schizophrenia were significantly more impaired than patients with nondeficit schizophrenia on each of the three factors (Table 2>). No between-group differences were found on the AIMS and Simpson-Angus scores.
Neuropsychological Evaluation
MANOVA revealed a significant overall group effect for neuropsychological test performance (Wilks’s lambda ratio=0.54; F=3.0, df=28, 234, p<0.00005).
ANOVA showed significantly lower IQ in patients with deficit schizophrenia versus patients with nondeficit schizophrenia (F=4.4, df=1, 106, p<0.04) and in each patient group versus healthy comparison subjects (Table 3). In light of these differences, statistical analyses for the remaining four neuropsychological function domains were carried out by ANCOVAs in which the general abilities domain was used as covariate.
Two-way repeated-measure ANCOVAs showed significant group effects or group-by-test interactions for each of the evaluated domains except visuospatial abilities (group effects seen for executive functions [F=4.7, df=2, 127, p<0.01] and explicit memory [F=8.0, df=2, 127, p<0.0007]; group-by-test interactions seen for executive functions [F=8.0, df=8, 512, p<0.00005], focused/sustained attention [F=16.4, df=2, 128, p<0.00005], and explicit memory [F=8.1, df=2, 128, p<0.0005]).
Follow-up ANCOVAs revealed no significant group effect for the comparisons between patients with deficit schizophrenia and patients with nondeficit schizophrenia. A significant group-by-test interaction was observed only for the focused/sustained attention domain (F=6.1, df=1, 103, p<0.01), for which follow-up analyses did not reveal a meaningful source. Comparisons between each patient group and healthy comparison subjects are shown in Table 3. On executive function tests, patients with nondeficit schizophrenia showed less flexibility and poorer ability to suppress interference and to form categories than healthy comparison subjects. Patients with deficit schizophrenia were significantly different from healthy comparison subjects only on the flexibility index. Both patient groups performed significantly worse than healthy comparison subjects on the WAIS-R Digit Symbol Substitution Test of the focused/sustained attention domain and on both tests of the explicit memory domain.
Stepwise Multiple Regression Analyses
To further investigate whether worse premorbid adjustment and more impaired neurological state are specifically associated with the deficit state after removing the effect of potential confounding variables, stepwise multiple regression analyses were performed on Premorbid Adjustment Scale and Neurological Evaluation Scale scores. The deficit/nondeficit categorization, the SANS/SAPS dimensions, age, education, duration of illness, the Simpson-Angus average score, and the chlorpromazine-equivalent doses were entered as independent variables.
The deficit/nondeficit categorization was significantly associated with Premorbid Adjustment Scale scores in childhood (F=13.8, df=1, 102, p<0.001; R2=0.12) and early adolescence (F=17.5, df=1, 102, p<0.001; R2=0.15). After removing this effect, the early adolescence Premorbid Adjustment Scale score did not show further significant associations, while the childhood Premorbid Adjustment Scale score was associated with education (F=6.7, df=1, 102, p<0.05; R2=0.05). The late adolescence Premorbid Adjustment Scale score was significantly associated with the negative symptom dimension (F=11.6, df=1, 102, p<0.001; R2=0.11); after removing this effect, it showed a further significant association with age (F=4.9, df=1, 102, p<0.05; R2=0.04).
For the Neurological Evaluation Scale factors, the Simpson-Angus average score was significantly associated with sensory integration (F=26.0, df=1, 102, p<0.001; R2=0.20), motor coordination (F=38.1, df=1, 102, p<0.001; R2=0.27), and complex motor sequences (F=21.2, df=1, 102, p<0.001; R2=0.17). After removing this effect, the sensory integration factor showed a further significant association with the negative symptom dimension (F=9.1, df=1, 102, p<0.01; R2=0.07), while the sequencing of complex motor acts factor was significantly associated with the deficit/nondeficit categorization (F=5.8, df=1, 102, p<0.05; R2=0.04).
Discussion
The pattern of historical, psychopathological, and neuropsychological characteristics previously reported in patients with deficit schizophrenia relative to those with the nondeficit subtype (7, 8, 10, 17) is mostly supported by the findings of the present study. This finding confirms that the deficit/nondeficit categorization, guided by the Schedule for the Deficit Syndrome after an appropriate training of the investigators, is replicable and reliable and allows the identification of patients with consistent clinical characteristics across independent studies. At odds with previous reports (9, 13), however, our patients with deficit schizophrenia and patients with nondeficit schizophrenia did not significantly differ on the expanded BPRS anxiety/depression factor.
Several important confounding variables were eliminated in this study by the design and the subject characteristics: patients with deficit schizophrenia and patients with nondeficit schizophrenia were matched for age and sex, had a comparable duration of illness, had been clinically stable for at least 3 months before the study procedures, and had a comparable severity of psychotic symptoms and disorganization. Furthermore, all the examiners, as well as interviewed patients and family members, were blind to the deficit/nondeficit categorization and were uninformed regarding the specific study aims and hypotheses. Different researchers performed the historical, psychopathological, neurological, and neuropsychological evaluations.
Our findings suggest that premorbid adjustment during childhood and early adolescence is poor in patients with deficit schizophrenia but not in patients with nondeficit schizophrenia. Actually, a significant association between poor premorbid adjustment and either negative symptoms or the deficit state has repeatedly been reported (8, 44, 45); however, to our knowledge, no attempt has been made to disentangle the respective contribution of negative symptoms and the deficit state to Premorbid Adjustment Scale score variance. Our data indicate that the association between the deficit state and poor premorbid adjustment is not due to the presence of more pronounced negative symptoms in the deficit group. These findings are consistent with Buchanan et al.’s hypothesis that patients with deficit schizophrenia represent a schizophrenic subgroup characterized by an early-onset disease process in which poor premorbid adjustment during childhood and early adolescence might represent the onset of deficit symptoms (8).
Patients with deficit schizophrenia exhibited more impairment on the Neurological Evaluation Scale than did those with nondeficit schizophrenia. The results of the stepwise multiple regression indicated that, after partialling out the influence of extrapyramidal symptoms, the deficit/nondeficit categorization was the only clinical variable entering the regression equation on the sequencing of complex motor acts factor, while the negative symptom dimension was associated with the sensory integration factor. The association of deficit schizophrenia with an impairment of sequencing of complex motor acts is in line with current hypotheses on the neural basis of the syndrome (19–21); both frontal and parietal deficits have been found in association with an impairment on sequencing of motor acts (46). At odds with our findings, a previous study has concluded that deficit schizophrenia is associated with an impairment of sensory integration, which was based on the results of separate multiple regressions that included either the deficit/nondeficit characterization or negative symptoms (18). The inclusion of different independent variables in the regression analysis might partly account for the discrepancy between the two studies. However, no direct comparison between our findings and those by Arango et al. (18) can be made. In their study, evaluation of the positive, negative, and disorganization symptoms was based on the BPRS (instead of the SANS and SAPS), only the conceptual disorganization was taken into account, and no evaluation of extrapyramidal symptoms was included.
As for neuropsychological results, in line with the Neurological Evaluation Scale findings that suggested frontoparietal dysfunction in subjects with deficit schizophrenia, when comparing patients with deficit and nondeficit schizophrenia, a significant group-by-test interaction was found only for the focused/sustained attention domain, which is thought to involve frontoparietal circuits.
When comparing each patient group with healthy subjects, patients with nondeficit schizophrenia exhibited more impairment in executive functions than did those with deficit schizophrenia. A greater impact of general abilities on the performance of subjects with deficit schizophrenia is actually responsible for such a finding (data not shown). These results are in agreement with Buchanan et al.’s finding of a strong influence of general abilities on neuropsychological performance in patients with deficit schizophrenia (15). Both the data of Buchanan et al. and our set of findings suggest that differences in intelligence/general abilities can no longer be regarded as just a “nuisance” requiring control. Low IQ has been found in association with negative symptoms, neurological signs, and poor premorbid adjustment and is increasingly recognized as a risk factor for schizophrenia (47, 48). Low IQ and other signs of cognitive and motor dysfunction have been repeatedly reported as childhood precursors of schizophrenia (48–51), although their presence seems to characterize only a minority of children who will develop schizophrenia (52, 53).
Deficit schizophrenia might represent a neurodevelopmental subtype of schizophrenia in which a significant behavioral and cognitive impairment since childhood compromises the development of basic capacities that contribute to subsequent cognitive and social functioning.
 |
 |
 |
Presented in part at the 154th annual meeting of the American Psychiatric Association, New Orleans, May 5–10, 2001. Received Aug. 13, 2001; revision received Jan. 7, 2002; accepted Jan. 14, 2002. From the University of Naples Department of Psychiatry; the University of L’Aquila Department of Psychiatry, L’Aquila, Italy; the University of Milan Department of Psychiatry, Milan, Italy; and the University of Pisa Department of Psychiatry, Pisa, Italy. Address reprint requests to Dr. Galderisi, Department of Psychiatry, University of Naples SUN, Largo Madonna delle Grazie, I-80138 Naples, Italy; [email protected] (e-mail). Supported by grant 9806499118 from the Italian Ministry of University and Scientific Research. The authors thank Drs. Paola Bucci, Luca Arduini, Nicolò Baldini, Massimiliano Bustini, Alberto Caputo, Paolo Cassano, Luca De Peri, Giuseppe Piegari, Osvaldo Rinaldi, Federico Soldani, and Paolo Stratta for their collaboration in data collection and analysis.
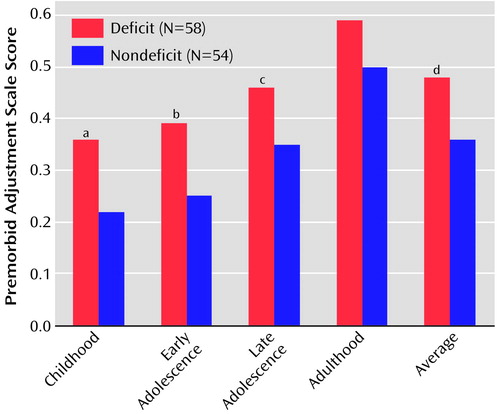
Figure 1. Premorbid Adjustment Scale Scores of Patients With Deficit Schizophrenia and Patients With Nondeficit Schizophrenia
aSignificantly worse premorbid adjustment seen in patients with deficit schizophrenia (F=14.9, df=1, 104, p=0.0002).
bSignificantly worse premorbid adjustment seen in patients with deficit schizophrenia (F=18.1, df=1, 104, p=0.00005).
cSignificantly worse premorbid adjustment seen in patients with deficit schizophrenia (F=7.5, df=1, 100, p=0.007).
dSignificantly worse premorbid adjustment seen in patients with deficit schizophrenia (F=16.9, df=1, 104, p=0.0001).
1. Maj M: Critique of the DSM-IV operational diagnostic criteria for schizophrenia. Br J Psychiatry 1998; 172:458-460Crossref, Medline, Google Scholar
2. Tsuang MT, Stone WS, Faraone SV: Toward reformulating the diagnosis of schizophrenia. Am J Psychiatry 2000; 157:1041-1050Link, Google Scholar
3. Carpenter WT Jr, Buchanan RW, Kirkpatrick B, Tamminga CA, Wood F: Strong inference, theory falsification, and the neuroanatomy of schizophrenia. Arch Gen Psychiatry 1993; 50:825-831Crossref, Medline, Google Scholar
4. Carpenter WT Jr, Heinrichs DW, Wagman AMI: Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry 1988; 145:578-583Link, Google Scholar
5. Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT Jr: A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry 2001; 58:165-171Crossref, Medline, Google Scholar
6. Amador XF, Kirkpatrick B, Buchanan RW, Carpenter WT, Marcinko L, Yale SA: Stability of the diagnosis of deficit syndrome in schizophrenia. Am J Psychiatry 1999; 156:637-639Link, Google Scholar
7. Fenton WS, McGlashan TH: Antecedents, symptom progression, and long-term outcome of the deficit syndrome in schizophrenia. Am J Psychiatry 1994; 151:351-356Link, Google Scholar
8. Buchanan RW, Kirkpatrick B, Heinrichs DW, Carpenter WT Jr: Clinical correlates of the deficit syndrome of schizophrenia. Am J Psychiatry 1990; 147:290-294Link, Google Scholar
9. Kirkpatrick B, Ram R, Bromet E: The deficit syndrome in the Suffolk County Mental Health Project. Schizophr Res 1996; 22:119-126Crossref, Medline, Google Scholar
10. Dollfus S, Germain-Robin S, Chabot B, Brazo P, Delamillieure P, Langlois S, van der Elst A, Campion D, Petit M: Family history and obstetric complications in deficit and nondeficit schizophrenia: preliminary results. Eur Psychiatry 1998; 13:270-272Crossref, Medline, Google Scholar
11. Kirkpatrick B, Castle D, Murray RM, Carpenter WT: Risk factors for the deficit syndrome of schizophrenia. Schizophr Bull 2000; 26:233-242Crossref, Medline, Google Scholar
12. Ross DE, Kirkpatrick B, Karkowski LM, Straub RE, MacLean CJ, O’Neill FA, Compton AD, Murphy B, Walsh D, Kendler KS: Sibling correlation of deficit syndrome in the Irish Study of High-Density Schizophrenia Families. Am J Psychiatry 2000; 157:1071-1076Link, Google Scholar
13. Kirkpatrick B, Amador XF, Yale SA, Bustillo JR, Buchanan RW, Tohen M: The deficit syndrome in the DSM-IV Field Trial, part II: depressive episodes and persecutory beliefs. Schizophr Res 1996; 20:79-90Crossref, Medline, Google Scholar
14. Buchanan RW, Breier A, Kirkpatrick B, Ball P, Carpenter WT Jr: Positive and negative symptom response to clozapine in schizophrenic patients with and without the deficit syndrome. Am J Psychiatry 1998; 155:751-760Link, Google Scholar
15. Buchanan RW, Strauss ME, Kirkpatrick B, Holstein C, Breier A, Carpenter WT Jr: Neuropsychological impairments in deficit vs nondeficit forms of schizophrenia. Arch Gen Psychiatry 1994; 51:804-811Crossref, Medline, Google Scholar
16. Bryson G, Whelahan HA, Bell M: Memory and executive impairments in deficit syndrome schizophrenia. Psychiatry Res 2001; 102:29-37Crossref, Medline, Google Scholar
17. Putnam KM, Harvey PD: Cognitive impairment and enduring negative symptoms: a comparative study of geriatric and nongeriatric schizophrenia patients. Schizophr Bull 2000; 26:867-878Crossref, Medline, Google Scholar
18. Arango C, Kirkpatrick B, Buchanan RW: Neurological signs and the heterogeneity of schizophrenia. Am J Psychiatry 2000; 157:560-565Link, Google Scholar
19. Tamminga CA, Thaker GK, Buchanan RW, Gao X, Shirakawa O, Buchanan R, Alphs LD, Carpenter WT, Chase T: Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch Gen Psychiatry 1992; 49:522-530Crossref, Medline, Google Scholar
20. Carpenter WT Jr, Lathi AC, Holcomb HH, Zhao M, Buchanan RW, Tamminga CA: Frontal and parietal blood flow activation during an auditory task differentiate schizophrenic patients with and without primary negative symptoms. Abstracts of the Society for Neuroscience 1996; 22:676Google Scholar
21. Heckers S, Goff D, Schacter DL, Savage CR, Fischman AJ, Alpert NM, Rauch SL: Functional imaging of memory retrieval in deficit vs nondeficit schizophrenia. Arch Gen Psychiatry 1999; 56:1117-1123Crossref, Medline, Google Scholar
22. Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT Jr: The Schedule for the Deficit Syndrome: an instrument for research in schizophrenia. Psychiatry Res 1989; 30:119-123Crossref, Medline, Google Scholar
23. DeLisi LE, Razi K, Stewart J, Relja M, Shields G, Smith AB, Wellman N, Larach VW, Loftus J, Vita A, Comazzi M, Crow TJ: No evidence for a parent-of-origin effect detected in the pattern of inheritance of schizophrenia. Biol Psychiatry 2000; 48:706-709Crossref, Medline, Google Scholar
24. Owen MJ, Lewis SW, Murray RM: Obstetric complications and schizophrenia: a computed tomographic study. Psychol Med 1988; 18:331-339Crossref, Medline, Google Scholar
25. Cannon-Spoor HE, Potkin SG, Wyatt RJ: Measurement of premorbid adjustment in schizophrenia. Schizophr Bull 1982; 8:470-484Crossref, Medline, Google Scholar
26. Ventura J, Green M, Shaner A, Liberman RP: Training and quality assurance on the BPRS: “the drift busters.” Int J Methods Psychiatr Res 1993; 3:221-224Google Scholar
27. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1981Google Scholar
28. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984Google Scholar
29. Strauss JS, Carpenter WT: The prediction of outcome in schizophrenia, I: characteristics of outcome. Arch Gen Psychiatry 1972; 27:739-746Crossref, Medline, Google Scholar
30. Buchanan RW, Heinrichs DW: The Neurological Evaluation Scale (NES): a structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Res 1988; 27:335-350Crossref, Google Scholar
31. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 534-537Google Scholar
32. Simpson GM, Angus JWS: A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 1970; 212:11-19Crossref, Medline, Google Scholar
33. Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G: Wisconsin Card Sorting Test Manual. Odessa, Fla, Psychological Assessment Resources, 1993Google Scholar
34. Maj M, D’Elia L, Satz P, Janssen R, Zaudig M, Uchiyama C, Starace F, Galderisi S, Chervinsky A: Evaluation of two new neuropsychological tests designed to minimize cultural bias in the assessment of HIV-1 seropositive persons: a WHO study. Arch Clin Neuropsychol 1993; 8:123-135Crossref, Medline, Google Scholar
35. Reitan RM, Wolfson D: The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation, 2nd ed. Tucson, Ariz, Neuropsychology Press, 1993Google Scholar
36. Rutschmann J, Cornblatt B, Erlenmeyer-Kimling L: Sustained attention in children at risk for schizophrenia: report on a continuous performance test. Arch Gen Psychiatry 1977; 34:571-575Crossref, Medline, Google Scholar
37. Benton AL, Sivan AB, Hamsher Kd, Varney NR, Spreen O: Judgment of Line Orientation, in Contributions to Neuropsychological Assessment: A Clinical Manual. New York, Oxford University Press, 1994, pp 44-54Google Scholar
38. Liddle PF: Schizophrenic syndromes, cognitive performance and neurological dysfunction. Psychol Med 1987; 17:49-57Crossref, Medline, Google Scholar
39. Liddle PF, Barnes TRE: Syndromes of chronic schizophrenia. Br J Psychiatry 1990; 157:558-561Crossref, Medline, Google Scholar
40. Peralta V, de Leon J, Cuesta MJ: Are there more than two syndromes in schizophrenia? a critique of the positive-negative dichotomy. Br J Psychiatry 1992; 161:335-343Crossref, Medline, Google Scholar
41. Galderisi S, Mucci A, Mignone ML, Bucci P, Maj M: Hemispheric asymmetry and psychopathological dimensions in drug-free patients with schizophrenia. Int J Psychophysiol 1999; 34:293-301Crossref, Medline, Google Scholar
42. Overall JE: The Brief Psychiatric Rating Scale in psychopharmacology research, in Psychological Measurements in Psychopharmacology: Modern Problems in Pharmacopsychiatry, vol 7. Edited by Pichot P, Olivier-Martin R. Basel, Switzerland, S Karger, 1974, pp 67-78Google Scholar
43. Lezak MD: Neuropsychological Assessment, 2nd ed. New York, Oxford University Press, 1983Google Scholar
44. Andreasen NC, Olsen S: Negative vs positive schizophrenia: definition and validation. Arch Gen Psychiatry 1982; 39:789-794Crossref, Medline, Google Scholar
45. Pogue-Geile MF, Harrow M: Negative symptoms in schizophrenia: their longitudinal course and prognostic importance. Schizophr Bull 1985; 11:427-439Crossref, Medline, Google Scholar
46. McCarthy RA, Warrington EK: Cognitive Neuropsychology: A Clinical Introduction. San Diego, Academic Press, 1990Google Scholar
47. Addington J, Addington D, Maticka-Tyndale E: Cognitive functioning and positive and negative symptoms in schizophrenia. Schizophr Res 1991; 5:123-134Crossref, Medline, Google Scholar
48. Jones P, Done DJ: From birth to onset: a developmental perspective of schizophrenia in two national birth cohorts, in Neurodevelopment and Adult Psychopathology. Edited by Keshavan MS, Murray RM. Cambridge, UK, Cambridge University Press, 1997, pp 119-136Google Scholar
49. David AS, Malmberg A, Brandt L, Allebeck P, Lewis G: IQ and risk for schizophrenia: a population based cohort study. Psychol Med 1997; 27:1311-1323Crossref, Medline, Google Scholar
50. Fish B, Marcus J, Hans SL, Auerbach JG, Perdue S: Infants at risk for schizophrenia: sequelae of a genetic neurointegrative defect: a review and replication analysis of pandysmaturation in the Jerusalem Infant Development Study. Arch Gen Psychiatry 1992; 49:221-235Crossref, Medline, Google Scholar
51. Walker EF, Savoie T, Davis D: Neuromotor precursors of schizophrenia. Schizophr Bull 1994; 20:441-451Crossref, Medline, Google Scholar
52. Torrey EF, Bowler AE, Taylor EH, Gottesman II: Schizophrenia and Manic Depressive Disorder. New York, Basic Books, 1994Google Scholar
53. Neumann CS, Grimes K, Walker EF, Baum K: Developmental pathways to schizophrenia: behavioral subtypes. J Abnorm Psychol 1995; 104:558-566Crossref, Medline, Google Scholar



