Higher Levels of CSF Homovanillic Acid in Recently Abstinent Cocaine-Dependent Patients
Abstract
OBJECTIVE: The authors examined an index of dopaminergic neurotransmission in recently abstinent cocaine-dependent patients. METHOD: CSF concentrations of the dopamine metabolite homovanillic acid (HVA) were determined in 30 recently abstinent cocaine-dependent patients and 69 healthy comparison subjects. RESULTS: The cocaine-dependent patients had a significantly higher mean concentration of CSF HVA than did the healthy comparison group. CONCLUSIONS: Recently abstinent cocaine-dependent patients may show dysregulation of the central dopaminergic system.
The main addicting properties of cocaine are related to its alteration of dopaminergic and other neurotransmission in the brain reward systems (1). One clinical strategy to examine central dopaminergic neurotransmission in humans is to determine by means of lumbar puncture CSF concentrations of the major dopamine metabolite homovanillic acid (HVA). This strategy has been extensively used in patients with other psychiatric disorders. However, as far as we are aware, there has only been one study that has assessed CSF HVA concentrations in cocaine-dependent patients (2). In that small study, no significant difference in CSF HVA was reported between nine cocaine-dependent patients and nine comparison subjects (2). However, the cocaine-dependent patients in that study were long-term inpatients who had been abstinent for an average of 28 weeks. Therefore, it was decided to examine CSF HVA in more recently abstinent cocaine-dependent patients.
Method
Thirty cocaine-dependent patients who had been admitted to the locked ward of the Substance Abuse Treatment Program (Department of Veterans Affairs, New Jersey Healthcare System, East Orange Campus) were studied. Patients were screened with the Structured Clinical Interview for DSM-IV (SCID) (3). Patients were enrolled in the study if they met DSM-IV criteria for cocaine dependence and identified cocaine as their illicit drug of first choice and did not currently meet dependence criteria for another substance or criteria for an axis I major psychiatric disorder during their lifetime. Patients with a medical disorder or taking any medication that could affect brain function were also excluded. After complete description of the study, written informed consent was obtained. When patients were free of illicit drugs, alcohol, and medications, a lumbar puncture was performed between the fourth and fifth lumbar vertebrae before 9:00 a.m. with the patient fasting and in the lateral decubitus position. The first 10 cc of CSF was collected as a pool, mixed, and aliquotted into tubes and stored in a refrigerator at –80°C until assayed.
Sixty-nine healthy men were also studied as a comparison group. They were recruited at Duke University in North Carolina through advertisements for a study on the relationship of brain function to cardiovascular reactivity. They were screened with the SCID to exclude those with psychiatric or medical disorders or current medication use. After providing informed consent, subjects were admitted to the General Clinical Research Center at Duke University Medical Center. There they underwent lumbar puncture performed by a board-certified anesthesiologist. Ten to 12 cc of CSF were obtained, mixed, and then separated into aliquots and frozen at –80°C until later assay. All CSF HVA assays were performed in the Duke University laboratory of one of the authors (C.K.) by using high-pressure liquid chromatography with electrochemical detection. Data were collected with a computer-based system and quantified with the use of internal standard and external standard curves. Sensitivity of the assay was 0.5 ng/sample.
Student’s t tests were used in the statistical analyses.
Results
All 30 cocaine-dependent patients were male. Their mean age was 40.2 years (SD=4.6); 22 were African American, six were Caucasian, and two were Hispanic. Their mean duration of cocaine abuse was 11.7 years (SD=5.4). The mean time free of all illicit drugs at the time of lumbar puncture was 28.6 days (SD=43.2, range=8–250). The 69 healthy comparison subjects were also all male. Their age range was 18 to 49 years; 34 were African American, and 35 were Caucasian.
As seen in Figure 1, the recently abstinent cocaine-dependent patients had a significantly higher mean concentration of CSF HVA than did the healthy comparison group (mean=33.6 ng/ml [SD=13.8] versus mean=25.4 ng/ml [SD=11.3], respectively). Results of an analysis of covariance with age and race as covariates also showed significantly higher CSF HVA in the cocaine-dependent patients than in the comparison subjects (F=3.92, df=3, 95, p<0.04). There was no significant correlation between CSF HVA and the number of days abstinent.
Discussion
Recently abstinent cocaine-dependent patients were found to have significantly higher CSF concentrations of the dopamine metabolite HVA than did a healthy comparison group. Dopamine is thought to be central to the reinforcing effects of cocaine (1). Cocaine binds to the dopamine transporter, and the resulting blockade of dopamine reuptake in the nucleus accumbens and brain reward systems is thought to underlie the euphoric effects of cocaine (1).
Recent brain imaging studies of the dopamine transporter have used the iodinated radioligand [123I]β-carbomethoxy-3 β-(4-iodophenyl) tropane (β-CIT) because it has structural similarities to cocaine. Single photon emission computed tomography studies that have used this ligand in recently abstinent cocaine-dependent patients have shown elevated striatal dopamine transporters in cocaine abusers relative to healthy comparison subjects (4). The authors suggested that these increases may be secondary to the chronic dopamine reuptake blockade effect of the cocaine abuse. Similarly, postmortem studies of cocaine abusers have reported marked increases in the number of striatal dopamine transporter sites relative to those of comparison subjects (5). Positron emission tomography studies have shown changes in dopamine receptor binding in recently abstinent cocaine-dependent patients (6). Thus, complex adaptational changes in central dopamine systems may lead to higher levels of CSF HVA in recently abstinent patients.
Other strengths of the present study include the fact that all subjects were male and inpatients on a locked ward. The subjects had thrice weekly negative urine screens for illicit drugs, and they were usually studied during the third week of their 3-week admission. Also, the patients were carefully chosen to form a homogenous group of patients without current axis I comorbidity and with only cocaine dependence. Patients with other current substance dependence, or taking any medication with a central action, were excluded.
We studied recently abstinent cocaine-dependent patients to compare the results with those of Knoblich et al. (2), who had found no significant difference in CSF HVA levels between cocaine-dependent patients and healthy comparison subjects. Their cocaine-dependent patients were long-term inpatients that had been abstinent from cocaine for an average of 28 weeks, whereas the mean length of abstinence in the present study was 28 days. Although cocaine withdrawal may be associated with age-related changes in central dopamine systems, in the present study there were no significant correlations between length of abstinence and CSF HVA level, even after we controlled for age (4). Although the groups were studied at two sites, all CSF HVA assays were performed in the same high-performance liquid chromatography laboratory. Studies have reported that smoking, height, and body mass index may influence CSF HVA concentrations, but since these variables were not recorded, this could not be examined (7–9).
Received April 17, 2001; revisions received Sept. 21 and Nov. 28, 2001; accepted Dec. 18, 2001. From the Psychiatry Service, Department of Veterans Affairs, New Jersey Healthcare System; and Duke University Medical School, Durham, N.C. Address reprint requests to Dr. Roy, Psychiatry Service (116A), Department of Veterans Affairs, New Jersey Healthcare System, 385 Tremont Ave., East Orange, NJ 07018. Supported in part by grant DA-10336 from the National Institute on Drug Abuse (Dr. Roy) and by grant HL-36587 from the National Heart, Lung, and Blood Institute (Dr. Williams).
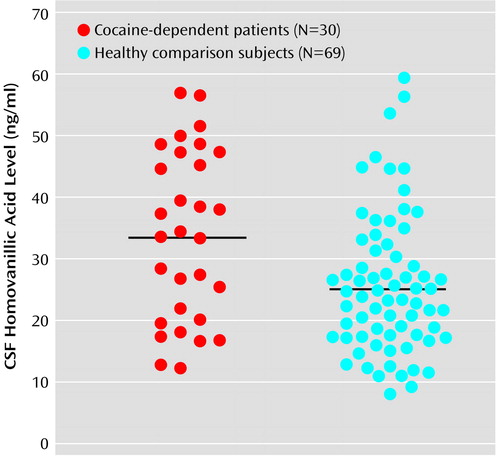
Figure 1. CSF Homovanillic Acid Levels in Recently Abstinent Cocaine-Dependent Patients and Healthy Comparison Subjectsa
aSignificantly higher levels of CSF homovanillic acid were seen in the cocaine-dependent patients (t=3.12, df=97, p<0.001). Horizontal bars indicate mean levels.
1. Withers N, Pulvirenti L, Koob G, Grillin C: Cocaine abuse and dependence. J Clin Psychopharmacol 1995; 15:63-78Crossref, Medline, Google Scholar
2. Knoblich G, Curtis D, Faustman W, Zarcone V, Stewart S, Mefford I, King R: Increased CSF HVA with craving in long-term abstinent cocaine abusers. Biol Psychiatry 1992; 32:96-100Crossref, Medline, Google Scholar
3. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-IV (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
4. Malison RT, Best SE, van Dyck CH, McCance EF, Wallace EA, Laruelle M, Baldwin RM, Seibyl JP, Price LH, Kosten TR, Innis RB: Elevated striatal dopamine transporters during acute cocaine abstinence as measured by [123I]β-CIT SPECT. Am J Psychiatry 1998; 155:832-834Abstract, Google Scholar
5. Little K, McLaughlin D, Zhang L, McFinton P, Dalack G, Cook E, Cassin B, Watson S: Brain dopamine transporter messenger RNA and binding sites in cocaine users. Arch Gen Psychiatry 2000; 55:793-799Crossref, Google Scholar
6. Volkow ND, Fowler JS, Wolff AP, Schlyer D, Shiue CY, Alpert R, Dewey SL, Logan J, Bendriem B, Christman D: Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry 1990; 147:719-724Link, Google Scholar
7. Geracioti TD Jr, West SA, Baker DG, Hill KK, Ekhator NN, Wortman MD, Keck PE Jr, Norman AB: Low CSF concentration of a dopamine metabolite in tobacco smokers. Am J Psychiatry 1999; 156:130-132Link, Google Scholar
8. Blennow K, Wallin A, Gottfries C, Karlsson I, Mansson J, Skoog I, Wikkelso C, Svennerholm L: Cerebrospinal fluid monoamine metabolites in 114 healthy individuals 18-88 years of age. Eur Neuropsychopharmacol 1993; 3:55-61Crossref, Medline, Google Scholar
9. Jonsson E, Sedvall G, Brene S, Gustavsson JP, Geijer T, Terenius L, Crocq MA, Lannfelt L, Tylec A, Sokoloff P, Schwartz JC, Wiesel FA: Dopamine-related genes and their relationship to monoamine metabolites in CSF. Biol Psychiatry 1996; 40:1032-1043Crossref, Medline, Google Scholar



