A Genome-Wide Scan for Linkage to Chromosomal Regions in 382 Sibling Pairs With Schizophrenia or Schizoaffective Disorder
Abstract
OBJECTIVE: Some genome-wide scans and association studies for schizophrenia susceptibility genes have yielded significant positive findings, but there is disagreement between studies on their locations, and no mutation has yet been found in any gene. Since schizophrenia is a complex disorder, a study with sufficient power to detect a locus with a small or moderate gene effect is necessary. METHOD: In a genome-wide scan of 382 sibling pairs with a diagnosis of schizophrenia or schizoaffective disorder, 396 highly polymorphic markers spaced approximately 10 centimorgans apart throughout the genome were genotyped in all individuals. Multipoint nonparametric linkage analysis was performed to evaluate regions of the genome demonstrating increased allele sharing, as measured by a lod score. RESULTS: Two regions with multipoint maximum lod scores suggesting linkage were found. The highest lod scores occurred on chromosome 10p15-p13 (peak lod score of 3.60 at marker D10S189) and the centromeric region of chromosome 2 (peak lod score of 2.99 at marker D2S139). In addition, a maximum lod score of 2.00 was observed with marker D22S283 on chromosome 22q12, which showed evidence of an imprinting effect, whereby an excess sharing of maternal, but not paternal, alleles was present. No evidence of linkage was obtained at several locations identified in previous studies, including chromosomes 1q, 4p, 5p-q, 6p, 8p, 13q, 15p, and 18p. CONCLUSIONS: The findings of this large genome-wide scan emphasize the weakness and fragility of linkage reports on schizophrenia. No linkage appears to be consistently replicable across large studies. Thus, it has to be questioned whether the genetic contribution to this disorder is detectable by these strategies and the possibility raised that it may be epigenetic, i.e., related to gene expression rather than sequence variation. Nevertheless, the positive findings on chromosome 2, 10, and 22 should be pursued further.
Schizophrenia affects approximately 1% of the general population if considered as a spectrum of genetically related clinical diagnoses (1). Despite numerous biological studies, no underlying inherited mechanism has been found. As polymorphic DNA markers and the laboratory techniques for high throughput linkage analyses have become available over the last 20 years, application of this strategy to finding genes for schizophrenia has been rigorously pursued by several research laboratories. However, numerous reports suggestive of linkage to specific chromosomal regions have contributed to widespread uncertainty concerning the significance of these diverse findings (2–13). Most recently, there have been reports of significant linkages on chromosomes 13q and 1q (5–7, 13–15). Despite all these efforts, at present there have been no convincing reports of any mutation in a schizophrenia susceptibility gene that demonstrate a high degree of statistical significance and/or an alteration in function.
We previously reported a genome-wide scan performed with 70 families having at least two affected siblings, at least one of whom had a diagnosis of schizophrenia (16). No genome-wide significance was observed, but lod scores above 2.0 or a p value of <0.01 were seen for chromosomes 1q22-q23, 2p14-p13, 4pter-cen, 5p15, 10q22-q24, 11pter-p11, 12p13-q24, 13q12-q13, 16q22.1, and 22q11. The present study was an expansion of the previous genome screen with the number of families increased to 309. We previously published negative results for this cohort on chromosomes 8, 13, and X, in response to earlier reports of linkage on these chromosomes (17, 18). The present manuscript summarizes the entire genome-wide scan and reports one significant and one suggestive region of genome-wide significance, according to the criteria for linkage of Lander and Kruglyak (19), but fails to confirm a number of previous reports of linkage. The present findings suggest that a critical reevaluation of the linkage approach is warranted.
Method
Subjects and Clinical Procedures
Families with schizophrenia or schizoaffective disorder in at least two siblings (N=309) were identified over a period beginning in 1985 to the present. These originated from five separate geographic collection centers: 213 families from the United States (based at Stony Brook, N.Y.), 50 from the United Kingdom (Oxford), 33 from Italy (Milan), 11 from Chile (Santiago), and two from Belgium (Leuven). The U.S./U.K. families were predominantly of Northern European descent. Details of all clinical procedures for this cohort have been previously published (16, 20, 21), as have pedigree structures (18, 22). Identification of the families and clinical evaluative and diagnostic procedures were similar in all locations. Diagnoses were made by the first author (L.E.D.) and other locally trained diagnosticians. Methods of recruiting included catchment area screening, systematic contact with health professionals at hospital and outpatient facilities, and advertisement through local and national support organizations for families of mentally ill persons (i.e., the National Alliance for the Mentally Ill in the United States and SANE [Schizophrenia A National Emergency] in the United Kingdom.) All individuals participating in this study gave written consent after receiving an explanation of the study procedures and their implications. Identical consent procedures were used in all five countries, and each center was given approval with Single Project Assurance status by the Office of Protection From Research Risks of the U.S. Department of Health and Human Services.
Diagnoses were made by using DSM-III-R criteria on the basis of structured interviews, review of medical records from all hospitalizations or other relevant treatment, and structured information obtained from at least one reliable family member about each individual. From 1985 to 1994 a modified Schedule for Affective Disorder and Schizophrenia (SADS) interview (23) combined with the Structured Interview for Personality Disorders (24) was used. After 1994, these forms were replaced by the newer, comprehensive Diagnostic Interview for Genetic Studies (25), and many of the ill individuals in previously obtained families were reinterviewed with this instrument. The SADS and Diagnostic Interview for Genetic Studies were translated into the appropriate language (i.e., Italian or Spanish) by professional medical translators and back checked. All foreign language interviews were summarized and translated into English before sending them to the Stony Brook site for diagnoses. The physicians and other professionals who performed the clinical evaluations (typically two individuals per site) were trained in these procedures by the first author (L.E.D.), and all underwent periodic diagnostic reliability exercises to maintain consistency between centers. Two independent diagnoses (one made by L.E.D.) were made for each individual in the study. In cases of disagreement between the diagnosing clinicians, a third diagnostician was consulted, and final diagnoses were made by consensus after discussion. Eight families were eliminated from the final analyses because of genetic inconsistencies in at least one crucial member. Thus, 301 families remained. Of these, 294 families had at least two siblings who satisfied the criteria for schizophrenia or schizoaffective disorder (382 nonindependent sibling pairs) (Table 1). The remaining seven families were excluded from the present analyses because one of the affected individuals did not satisfy criteria for these two diagnoses. An initial genome-wide scan involving 70 of these families and using different markers was previously reported (16).
The question of how best to define the affected phenotype from clinical characteristics was considered. Family and adoption studies have shown a genetic relationship between schizophrenia and schizoaffective disorder, and there may not be a scientific basis for the distinction between the two diagnostic categories, since the majority of patients with schizophrenia display depressive symptoms sometime in the course of their illness (reviewed in reference 26). In addition, the diagnostic reliability of the distinction between schizophrenia and schizoaffective disorder is low (25). Thus, these diagnoses were combined into one affected category, with schizoaffective disorder considered as affected.
Genotyping
Three hundred ninety-six highly polymorphic microsatellite markers were genotyped in all families by using standard polymerase chain reaction procedures that incorporated multiplex fluorescent genotyping, as previously described (27). Markers were selected for genotyping on the basis of their heterozygosity and distance from each other in order to cover the entire genome. The markers had an average spacing of 10 centimorgans (cM) (range=0–23 cM) and generated an average genetic information content of 0.75.
Statistical Analyses
Power analyses were calculated to determine the likelihood of detecting linkage under various gene effect sizes by using study group sizes corresponding to the schizophrenia plus schizoaffective disorder phenotype in the sibships from the total group of families. The methods used to determine the estimated power have been described by Risch (28). Since these power calculations assumed fully informative matings and the average heterozygosity of our markers was approximately 75%, the values represented maximum power. In addition, since the markers were spaced in approximate 10-cM intervals, the power to detect linkage was calculated at a recombination fraction of 0.05 between marker and disease loci (on average, the maximum distance between a potential disease locus and a marker locus). Our study group size had approximately 98% power to detect linkage at a lod score threshold of 2.0 at a relatively small gene effect size as measured by a sibling relative risk (lambda-s) of 2.0 and 89% power to detect linkage at a lod score of 3.0 at the same gene effect size.
Analyses were performed by using a schizophrenia and schizoaffective disorder phenotype, excluding the broader diagnoses of psychosis not otherwise specified or schizophrenia spectrum personality disorder.
For all analyses, allele frequencies for each marker were calculated from the family genotype data. Each of the 396 markers in the genome scan in affected sibling pairs were analyzed by using both the 2-point and the multipoint nonparametric allele sharing tests in MAPMAKER/SIBS (29). Two-point lod scores were computed at each marker by summing the log likelihood ratio of the observed identity-by-descent allele sharing among affected sibling pairs to random Mendelian segregation of 0.25, 0.50, 0.25 for sharing 0, 1, or 2 alleles identity-by-descent, respectively. For multipoint analysis, lod scores were computed in a similar manner at 1-cM intervals along each chromosome. A weighted average (2 divided by the number of affected siblings) was used for families containing more than two affected siblings. Parent-of-origin allele sharing tests were carried out on chromosome 22 markers by using the sib-ibd module of ASPEX (30).
Parametric analyses were conducted for the two highest regions of significant or suggestive genome-wide linkage by using both recessive and dominant affecteds-only models. Two-point lod scores were calculated with the program MLINK of FASTLINK (31, 32) at increments of theta=0.05, starting at theta=0 and ending at theta=0.5. Zmax scores were estimated from the maximum lod score obtained in these iterations. In addition, 2-point lod scores were calculated by assuming heterogeneity, where both alpha (the proportion of linked families) and the lod score were iterated and theta was fixed at 0 by using the program GENEHUNTER (33).
Results
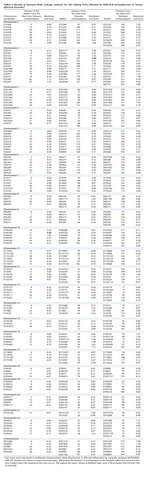
The multipoint maximum lod score between each marker interval is indicated for all markers included in the genome scan (Table 2). There was one region with significant and two with suggestive linkage in this genome scan (lod score greater than 2.2 [19]). The significant peak lod score occurred in 10p14 at marker D10S189 with a Zmax of 3.60. The second best linkage result occurred in the centromeric region of chromosome 2 with a peak lod score of 2.99 between markers D2S139 and D2S417. A second peak on chromosome 2 occurred approximately 17 cM distal in 2q12 between markers D2S160 and D2S2254, with a peak lod score of 2.73. Finally, the third best linkage result occurred in 3q27 between markers D3S1602 and D3S1580 with a peak lod score of 2.31. Other peak lod scores ≥2.0 were obtained on chromosome 12q22-q24 at marker D12S324 (Zmax=2.10) and on chromosome 22q12-q13 at D22S283 (Zmax=2.00). The multipoint maximum lod score curves for all chromosomes are shown in Figure 1. All of the corresponding lod scores and markers used are shown in Table 2.
Parametric lod score analyses were calculated for markers in the chromosome 2 and 10 linkage regions, testing both autosomal dominant and recessive models by using an affecteds-only analysis. The results are shown in Table 3. In the chromosome 10p region, the peak parametric lod scores were obtained by using a recessive model (disease allele frequency=0.0091, phenocopy rate=0.0006). The peak lod score under homogeneity occurred at D10S189, with a Zmax of 2.25 (theta=0.30). Under heterogeneity, the lod score increased to 4.07 at D10S189 (theta=0.00, alpha=0.17). In the centromeric region of chromosome 2, the highest lod score assuming homogeneity occurred at D2S286, with a Zmax of 2.79 (theta=0.30), also by using a recessive model. When the data were analyzed under heterogeneity, the highest lod score occurred at D2S2229, with a lod score of 3.33 (theta=0.00, alpha=0.12). In addition, under heterogeneity, marker D2S139 located 16 cM proximal to D2S2229 in 2q12 had a peak lod score of 2.52 (theta=0.00, alpha=0.14).
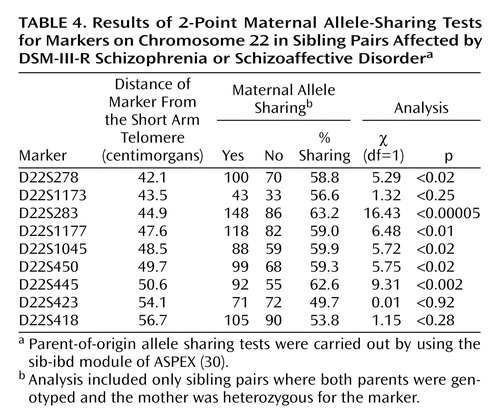
Because of the interest in a possible imprinting effect on chromosome 22q (Vallada and Collier [34]), we investigated both maternal and paternal sharing of alleles among affected sibling pairs. Results of a chi-square test suggested a significant excess sharing of maternal alleles at locus D22S283 (χ2=16.43, df=1, p=0.00005). The results of the 2-point maternal allele-sharing tests for all markers on chromosome 22 are shown in Table 4.
Discussion
Genetic linkage studies of schizophrenia are currently in progress in independent laboratories worldwide. The limiting factor for most investigators has been the lack of availability of a large number of families for analysis. Thus, there have been many reports of suggestive linkage, but very few with significant findings. The present report contributes results from one of the largest complete genome-wide scans of schizophrenia to date. Although our findings when viewed on their own yield some evidence of linkage—for example, significant linkage in chromosome 10p14 and a suggestion of linkage in chromosome 2p14-q12—the most striking feature is the failure to confirm a number of earlier claims of positive findings.
We first consider the apparent evidence for linkage from our own studies. In an initial genome-wide scan (16), chromosome 10p did not show a positive peak. However, others have previously reported 10p findings that suggest linkage to schizophrenia (9, 35, 36), and Schwab et al. (37) have presented data that phosphatidylinositol phosphate kinase on 10p is a candidate gene. However, like our findings for chromosome 2, the chromosome 10p findings are spread over a considerable distance. The finding in our present study (peak lod score of 3.60 at D10S189, 17cM from pter [the short-arm telomere]) is approximately 15–20 cM from the findings of Straub et al. (9) (maximum heterogeneity lod score of 1.95 in a recessive model). It is 25–30 cM from the peak multipoint lod score of 2.13 obtained by Schwab et al. (9), and at the short-arm end of the 60-cM band of positive nonparametric linkage scores of 1.70 to 3.36 from 10p13 to 10q23 reported by Faraone et al. (36) in the European-American pedigrees of the National Institute of Mental Health (NIMH) data set. These scores are thus modest in magnitude and variable in location. Moreover, other systematic genome scans give no support to linkage on chromosome 10. Moises et al. (38) in a two-stage approach in 70 pedigrees, Coon et al. (39) in a large Palaoan pedigree, Brzustowicz et al. (7) in 22 extended Canadian pedigrees, Williams et al. (40) in their two-stage analysis of 196 affected sibling pairs, and Kaufmann et al. (41) in African American pedigrees in the NIMH data all used markers with a resolution of 10 cM or less and failed to observe any positive peaks on 10p. Thus, there is little support in the literature for a conclusion that our multipoint lod score of 3.60 at D10S189 is evidence of a gene for schizophrenia on chromosome 10p14.
In our initial genome scan, we reported a peak lod score of 2.13 on chromosome 2p16.1 at D2S1337. In the current study, the expanded cohort had a peak lod score exceeding criteria for suggestive linkage as suggested by Lander and Kruglyak (19) in the centromeric region of chromosome 2 near the marker D2S417 (102 cM from pter) and second peak in 2q12 at D2S160 (118 cM from pter). Other studies of families with schizophrenia have reported suggestive linkage for this region, although the associations have not reached a high level of statistical significance (36, 38, 39, 42). Coon et al. (39) reported a peak positive score on chromosome 2 at D2S441, which is approximately 98 cM from pter, and Faraone et al. (36) reported a peak nonparametric linkage score of 2.41 on chromosome 2q12 at 104.5 cM from pter in a cohort of 43 nuclear families. Similarly, Levinson et al. (42) reported a peak nonparametric linkage score of 2.01 at D2S410 located in 2q14.1 approximately 115 cM from pter in 45 pedigrees. In one of the earliest reported genome-wide scans for schizophrenia susceptibility genes, Moises et al. (38) reported a nonparametric p value of 0.0001 at D2S135 located approximately 115 cM from pter in 2q12-2q14 in a group of Icelandic and international families. Brzustowicz et al. (6, 7), while noting genome-wide evidence of significant linkage on chromosomes 1q and 13q, found a peak score of 2.42 at D2S1400, approximately 28 cM from pter. In a study of 198 affected sibling pairs, Williams et al. (40) reported a wide peak on 2q at approximately 181 cM from pter, considerably distal to the peak in the present study. Kaufmann et al. (41) reported modest peaks (nonparametric linkage scores <1.5) at 40 cM and 220 cM in African American pedigrees from the NIMH genetics consortium, with no evidence of linkage around the centromere. Thus, although all of these studies have reported findings on chromosome 2, the peaks are of modest magnitude and are widely dispersed along the chromosome. Only one of these findings (39) comes within 4 cM of the present finding.
Chromosome 22q became a focus of attention after the report of Pulver et al. (3) suggesting linkage to this chromosome in schizophrenia and descriptions of schizophrenia-like symptoms in patients with velocardiofacial syndrome, which arises from a deletion in chromosome 22q11 (43). This syndrome and its relationship to schizophrenia have been extensively studied (44, 45). Some positive findings have been reported of linkage more distal to this region on 22q12 (3, 46, 47), and one report of a possible imprinting effect on this putative locus (linkage to maternally but not paternally transmitted alleles) in schizophrenia (48) is consistent with our findings. Nevertheless, our weakly positive lod score for this region in our initial scan of 70 families did not increase further when the cohort was substantially enlarged. In addition, no evidence of linkage in this region was reported by Kaufmann et al. (41), Faraone et al. (36), Williams et al. (40), or Brzustowicz et al. (7). Similarly, the chromosome 3 and 12 suggestive linkages in the present study have not been reported to be of significance in any previous publications on schizophrenia.
Other regions of potential interest in our initial screen (chromosomes 1q, 4p, 5p, 11p, 16q) were not supported by further evidence of linkage in the fourfold larger cohort in the present scan. Moreover, a number of regions within which specific claims for linkage have been made by other investigators on 5q (2), 6p 24-22 (e.g., reference 8), 13q (5, 6), and 1q (7, 14, 15) with small numbers of families have not shown linkage in the present group of 294 families. Two recent claims are of particular note. In 22 extended families, Brzustowicz et al. (7) reported a heterogeneity lod score of 6.5 with an alpha (proportion of linked families) of 75% for a region in chromosome 1q211-q22 and claimed that the linkage result “should provide sufficient power to allow the positional cloning of the underlying susceptibility gene.” Leonard and colleagues (48) reanalyzed data from the relatively small number of families from the NIMH consortium and obtained a lod score of 4.43 at 45.7 cM from the 15p telomere in a heterogeneity analysis with an alpha of 85%. If either of these claims were generalizable to a group of 294 families, we would expect to see lod scores substantially in excess of those reported in references 7 and 48. In fact, in our present study, we found no evidence of linkage to either of these regions. Although it is possible that some unusual skewness in the heterogeneity of our sample and those of others may explain our failure to replicate some previously reported findings, it is also possible that some of the reported linkages (or most) are false positive findings that will not lead to the location of a susceptibility gene.
Alternatively, it may be that the numbers of families with schizophrenia in all existing studies, including our own, are too small to replicate findings consistently. This argument has been supported by the simulated calculations of Goring and colleagues (49) suggesting that if several genes of small effect are involved, as many as 3,000 sibling pairs would be needed to replicate results consistently. Thus, many positive regions with susceptibility genes could be missed even in a group of families as large as our current study group, and unusually large effects may be falsely seen with a much smaller number of families and may be falsely interpreted as evidence for the presence of susceptibility genes.
It is likely that the scope and complexity of linkage analysis combined with the idiosyncrasy of small study group sizes and a natural enthusiasm for positive findings has led investigators to conclusions that cannot be generalized to schizophrenia as a whole. We and others have not yet found a gene for susceptibility to schizophrenia or any other psychosis, although there is hope for the future, given the continued advances in technology. A candidate gene approach that takes into account the key underlying pathology present in patients with long-term schizophrenia (including brain structural, cognitive, and other physiological defects) may be a more fruitful direction. The possibility that linkage or association to a candidate gene may not be present owing to a lack of sequence variation needs to be considered, and the alternative possibility that the anomaly is epigenetic and related to variability in gene expression should be considered. Although the present results on chromosomes 2, 10, and 22 are intriguing and worth further pursuit, we acknowledge that they may be only chance findings awaiting replication and the detection of candidate genes within these regions.
 |
 |
 |
 |
Received Sept. 13, 2001; revision received Jan 9, 2002; accepted Jan. 16, 2002. From the Department of Psychiatry, New York University; Axys Pharmaceuticals, La Jolla, Calif.; the University Department of Psychiatry, Warneford Hospital, Oxford, U.K.; the University of Chile, Santiago, Chile; the University of Milan, Milan, Italy; and Pfizer, Inc., Ann Arbor, Mich.Address reprint requests to Dr. DeLisi, Department of Psychiatry, New York University, Millhauser Laboratories, 550 First Ave., New York, NY 10016; [email protected] (e-mail). Supported by Warner-Lambert, Parke-Davis Pharmaceuticals Company, and NIMH grant MH-44245.
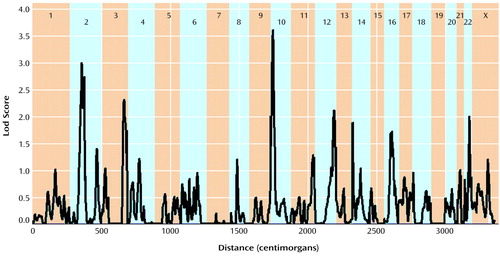
Figure 1. Multipoint Nonparametric Maximum Lod Scores in a Genome-Wide Linkage Analysis for 382 Sibling Pairs Affected by DSM-III-R Schizophrenia or Schizoaffective Disordera
aThe x axis represents distance within the entire genome.
1. Gottesman II, Shields J: Schizophrenia: The Epigenetic Puzzle, Cambridge, UK, Cambridge University Press, 1982Google Scholar
2. Sherrington R, Brynjolfsson J, Petursson H, Potter M, Dudleston K, Barraclough B, Wasmuth J, Dobbs M, Gurling H: Localization of a susceptibility locus for schizophrenia on chromosome 5. Nature 1988; 336:164-167Crossref, Medline, Google Scholar
3. Pulver AE, Karayiorgou M, Wolyniec PS, Lasseter V, Kasch L, Nestadt G, Antonarakis S, Housman D, Kazazian HH, Meyers D, Ott J, Lamacz M, Liang K-Y, Hanfelt J, Ulrich G, DeMarchi N, Ranu E, McHugh PR, Adler L, Thomas M, Carpenter WT, Manschreck T, Gordon CT, Kimberland M, Babb R, Puck J, Childs B: Sequential strategy to identify a susceptibility gene for schizophrenia: report of a potential linkage on chromosome 22q12-q12.1, part I. Am J Med Genet 1994; 54:36-43Crossref, Medline, Google Scholar
4. Pulver AE, Lasseter VK, Kasch L, Wolyniec P, Nestadt G, Blouin JL, Kimberland M, Babb R, Vourlis S, Chen H, Lalioti M, Morris MA, Karayiorgou M, Ott J, Meyers D, Antonarakis SE, Housman D, Kazazian HH: Schizophrenia: a genome scan targets chromosome 3p and 8p as potential sites of susceptibility genes. Am J Med Genet 1995; 60:252-260Crossref, Medline, Google Scholar
5. Blouin J-L, Dombroski BA, Nath SK, Lasseter VK, Wolyniec PS, Nestadt G, Thornquist M, Ullrich G, McGrath J, Kasch L, Lamacz M, Thomas MG, Gerrig C, Radhakrishna U, Snyder SC, Balk KG, Neufeld K, Swartz KL, DeMarchi N, Papadimitriou GN, Dikeus DG, Stefanis CN, Chakravarti A, Childs B, Pulver AE: Schizophrenia susceptibility loci on chromosome 13q32 and 8p21. Nat Genet 1998; 20:70-73Crossref, Medline, Google Scholar
6. Brzustowicz LM, Honer WG, Chow EW, Little D, Hogan J, Hodfkinson K, Bassett AS: Linkage of familial schizophrenia to chromosome 13q32. Am J Hum Genet 1999; 65:1096-1103Crossref, Medline, Google Scholar
7. Brzustowicz LM, Hodgkinson KA, Chow EW, Honer WG, Bassell AS: Location of a major susceptibility locus for familial schizophrenia on chromosome 1q21-q22. Science 2000; 288:678-682Crossref, Medline, Google Scholar
8. Straub RE, MacLean CJ, Walsh D, Kendler KS: Support for schizophrenia vulnerability loci on chromosomes 6p and 8p from Irish families. Cold Spring Harbor Symp Quant Biol 1996; 61:823-833Crossref, Medline, Google Scholar
9. Straub RE, MacLean CJ, Martin RB, Ma Y, Myakishev MV, Harris-Kerr C, Webb BT, O’Neill FA, Walsh D, Kendler KS: A schizophrenia locus may be located in region 10p15-p11. Am J Med Genet 1998; 81:296-301Crossref, Medline, Google Scholar
10. Schizophrenia Collaborative Linkage Group for Chromosomes 3, 6, and 8: Additional support for schizophrenia linkage findings on chromosomes 6 and 8: a multicenter study. Am J Med Genet 1996; 67:580-594Crossref, Medline, Google Scholar
11. DeLisi LE: A critical overview of recent investigations into the genetics of schizophrenia. Curr Opin Psychiatry 1999; 12:29-39Crossref, Google Scholar
12. DeLisi LE, Crow TJ: Chromosome Workshops 1998: current state of psychiatric linkage. Am J Med Genet 1999; 88:215-218Crossref, Medline, Google Scholar
13. DeLisi LE, Craddock NJ, Detera-Wadleigh S, Foroud T, Gejman P, Kennedy JL, Lendon C, Macciardi F, McKeon P, Mynett-Johnson L, Nurnberger JI Jr, Paterson A, Schwab S, Van Broeckhoven C, Wildenauer D, Crow TJ: Update on chromosomal locations for psychiatric disorders: report of the interim meeting of chromosome workshop chairpersons from the VIIth World Congress of Psychiatric Genetics, Monterey, California, October 14-18, 1999. Am J Med Genet 2000; 96:434-449Crossref, Medline, Google Scholar
14. Gurling HMD, Kalsi G, Blaveri E, McQuillin A, Read T, Murphy P, Butler R, Brynjolfsson J, Sigmundsson T, Petursson H, Curtis D: Initial genome wide parametric genetic linkage analysis of schizophrenia and schizophrenia spectrum disorders finds lod scores above 3.00 on four chromosomes at 1q22-23, 5q22-35, 8p21-23 and 11q14-24: a further lod above 3.00 at 4q21-31 was found within a single family (abstract). Mol Psychiatry 1999; 4(suppl 1):S4Google Scholar
15. Ekelund J, Lichtermann D, Hovatta I, Ellonen P, Suvisaari J, Terwilliger JD, Juvonen H, Varilo T, Arajarvi R, Kokko-Sahin ML, Lonnqvist J, Peltonen L: Genome-wide scan for schizophrenia in the Finnish population: evidence for a locus on chromosome 7q22. Hum Mol Genet 2000; 9:1049-1057Crossref, Medline, Google Scholar
16. Shaw SH, Kelly M, Smith AB, Shields G, Hopkins PJ, Loftus J, Laval SH, Vita A, De Hert M, Cardon LR, Crow TJ, Sherrington R, DeLisi LE: A genome-wide screen for linkage to schizophrenia. Am J Med Genet 1998; 81:364-376Crossref, Medline, Google Scholar
17. DeLisi LE, Shaw S, Crow TJ, Shields G, Smith AB, Larach VW, Wellman N, Loftus J, Nathankumar B, Razi K, Kushner M, Stewart J, Vita A, Comazzi M, Sherrington R: Lack of evidence for linkage to chromosomes 13 and 8 for schizophrenia and schizoaffective disorder. Am J Med Genet 2000; 96:235-239Crossref, Medline, Google Scholar
18. DeLisi LE, Shaw S, Sherrington R, Nanthakumar B, Shields G, Smith AB, Wellman N, Larach VW, Loftus J, Razi K, Stewart J, Comazzi M, Vita A, De Hert M, Crow TJ: Failure to establish linkage on the X chromosome in 301 families with schizophrenia or schizoaffective disorder. Am J Med Genet 2000; 96:335-341Crossref, Medline, Google Scholar
19. Lander E, Kruglyak L: Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet 1995; 11:241-247Crossref, Medline, Google Scholar
20. DeLisi LE, Devoto M, Lofthouse R, Poulter M, Smith A, Shields G, Bass N, Chen G, Vita A, Morganti C, Ott J, Crow TJ: Search for linkage to schizophrenia on the X and Y chromosomes. Am J Med Genet 1994; 54:113-121Crossref, Medline, Google Scholar
21. Garner C, Kelly M, Cardon L, Joslyn G, Carey A, LeDuc C, Lichter J, Harris T, Loftus J, Shields G, Comazzi M, Vita A, Smith AM, Dann J, Crow TJ, DeLisi LE: Linkage analyses of schizophrenia to chromosome 6p24-p22: an attempt to replicate. Am J Med Genet 1996; 67:595-610Crossref, Medline, Google Scholar
22. Crow TJ, DeLisi LE, Johnstone EC: Concordance by sex for psychosis is paternally inherited: evidence for a pseudoautosomal locus. Br J Psychiatry 1989; 155:92-97Crossref, Medline, Google Scholar
23. Spitzer RL, Endicott J: Schedule for Affective Disorders and Schizophrenia (SADS), 3rd ed. New York, New York State Psychiatric Institute, Biometrics Research, 1978Google Scholar
24. Pfohl B, Blum N, Zimmerman M, Stangl D: Structured Interview for DSM-III-R Personality, Revised (SIDP-R). Iowa City, University of Iowa College of Medicine, Department of Psychiatry, 1989Google Scholar
25. Nurnberger JI Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T (NIMH Genetics Initiative): Diagnostic Interview for Genetic Studies: rationale, unique features, and training. Arch Gen Psychiatry 1994; 51:849-859Crossref, Medline, Google Scholar
26. DeLisi LE: Depression in Schizophrenia. Washington, DC, American Psychiatric Press, 1992Google Scholar
27. Hall JM, Le Duc CA, Watson AR, Roter AH: An approach to high-throughput genotyping. Genome Res 1996; 6:781-790Crossref, Medline, Google Scholar
28. Risch N: Linkage strategies for genetically complex traits, II: the power of affected relative pairs. Am J Hum Genet 1990; 46:229-241Medline, Google Scholar
29. Kruglyak L, Daly MJ, Lander ES: Complete multipoint sib-pair analysis of qualitative and quantitative traits. Am J Hum Genet 1995; 57:439-454Medline, Google Scholar
30. Hinds DA, Risch N: The ASPEX package: affected sib-pair exclusion mapping. http://bioweb.pasteur.fr/docs/aspex/usage.htmlGoogle Scholar
31. Lathrop GM, Lalouel JM, Julier C, Ott J: Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci USA 1984; 81:3443-3446Crossref, Medline, Google Scholar
32. Cottingham R, Idury R, Schaffer A: Faster sequential genetic linkage computations. Am J Hum Genet 1993; 53:252-263Medline, Google Scholar
33. Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES: Parametric and non-parametric linkage analysis: a unified multipoint approach. Am J Hum Genet 1996; 58:1347-1363Medline, Google Scholar
34. Vallada HP, Collier DA: Genetics of schizophrenia—new findings, in Search for the Causes of Schizophrenia, vol IV: Balance of the Century. Edited by Gattaz WF, Hafner H. Berlin, Springer-Verlag, 1999, pp 181-189Google Scholar
35. Schwab SG, Hallmayer J, Albus M, Lerer B, Hanses C, Kanyas K, Segman R, Borrman M, Dreikorn B, Lichtermann D, Rietschel M, Trixler M, Maier W, Wildenauer DB: Further evidence for a susceptibility locus on chromosome 10p14-p11 in 72 families with schizophrenia by nonparametric linkage analysis. Am J Med Genet 1998; 81:302-307Crossref, Medline, Google Scholar
36. Faraone SV, Matise T, Svrakic D, Pepple J, Malaspina D, Suarez B, Hampe C, Zambuto CT, Schmitt K, Meyer J, Markel P, Lee H, Harkavy Friedman J, Kaufmann C, Cloninger CR, Tsuang MT: Genome scan of European-American schizophrenia pedigrees: results of the NIMH Genetics Initiative and Millenium Consortium. Am J Med Genet 1998; 81:290-295Crossref, Medline, Google Scholar
37. Schwab SG, Eckstein GN, Gable S, Halmeyer J, Lerer B, Albus M, Maier W, Wildenauer DB: Susceptibility locus for schizophrenia on 10p: searching for a candidate (abstract). Am J Med Genet 2000; 96:461Google Scholar
38. Moises HW, Yang L, Kristbjarnarson H, Wiese C, Byerley W, Macciardi F, Arolt V, Blackwood D, Liu X, Sjorgen B, Ashaueer HN, Hwu H-G, Jang K, Livesley WJ, Kennedy JL, Zoega T, Ivarsson O, Bui M-T, Yu M-H, Havsteen B, Commenges D, Weissenbach J, Schwinger E, Gottesman II, Pakstis AJ, Wetterberg L, Kidd KK, Helgason T: An international two-stage genome-wide search for schizophrenia susceptibility genes. Nat Genet 1995; 11:321-324Crossref, Medline, Google Scholar
39. Coon H, Myles-Worsley M, Tiobech J, Hoff M, Rosenthal J, Bennett P, Reimherr F, Wender P, Dale P, Polloi A, Byerley W: Evidence for a chromosome 2p13-14 schizophrenia susceptibility locus in families from Palau, Micronesia. Mol Psychiatry 1998; 3:521-527Crossref, Medline, Google Scholar
40. Williams NM, Rees MI, Holmans P, Norton N, Cardno AG, Jones LA, Murphy KC, Sanders RD, McCarthy G, Gray MY, Fenton I, McGuffin P, Owen MJ: A two-stage sib-pair genome scan of schizophrenia susceptibility genes in 196 affected sibling pairs. Hum Mol Genet 1999; 8:1729-1740Crossref, Medline, Google Scholar
41. Kaufmann CA, Suarez B, Malaspina D, Pepple J, Svrakic D, Markel PD, Meyer J, Zambuto CT, Schmitt K, Matise TC, Harkavy Friedman JM, Hampe C, Lee H, Shore D, Wynne D, Faraone SV, Tsuang MT, Cloninger CR: NIMH Genetics Initiative Millennium Schizophrenia Consortium: linkage analysis of African-American pedigrees. Am J Med Genet 1998; 81:282-289Crossref, Medline, Google Scholar
42. Levinson DF, Mahtani MM, Nancarrow DJ, Brown DM, Kruglyak L, Kirby A, Hayward NK, Crowe RR, Andreasen NC, Black DW, Silverman JM, Endicott J, Sharpe L, Mohs RC, Siever LJ, Walters MK, Lennon DP, Jones HL, Nertney DA, Daly MJ, Gladis M, Mowry BJ: Genome scan of schizophrenia. Am J Psychiatry 1998; 155:741-750Abstract, Google Scholar
43. Murphy KC, Jones LA, Owen MJ: High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry 1999; 56:940-945Crossref, Medline, Google Scholar
44. Karayiorgou M, Morris MA, Morrow B, Shprintzen RJ, Goldberg R, Borrow J, Gos A, Nestadt G, Wolyniec PS, Lasseter VK, Pulver AE: Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc Natl Acad Sci USA 1995; 92:7612-7616Crossref, Medline, Google Scholar
45. Bassett AS, Chow EW: 22q11 deletion syndrome: a genetic subtype of schizophrenia. Biol Psychiatry 1999; 46:882-891Crossref, Medline, Google Scholar
46. Gill M, Vallada H, Collier D, Sham P, Holmans P, Murray R, McGuffin P, Nanko S, Owen M, Antonarakis S, Housman D, Kazazian H, Nestadt G, Pulver AE, Straub RE, MacLean CJ, Walsh D, Kendler KS, DeLisi L, Polymeropoulos M, Coon H, Byerley W, Lofthouse R, Gershon E, Read CM (Schizophrenia Collaborative Linkage Group [Chromosome 22]): A combined analysis of D22S278 marker alleles in affected sib-pairs: support for a susceptibility locus for schizophrenia at chromosome 22q12. Am J Med Genet 1996; 67:40-45Crossref, Medline, Google Scholar
47. Coon H, Jensen S, Holik M, Hoff M, Myles-Worsley M, Reimherr F, Wender P, Waldo M, Freedman R, Leppert M, Byerley W: Genomic scan for genes predisposing to schizophrenia. Am J Med Genet 1994; 54:59-71Crossref, Medline, Google Scholar
48. Leonard S, Gault J, Moore T, Hopkins J, Robinson M, Oliney A, Adler LE, Cloninger CR, Kaufmann CA, Tsuang MT, Faraone SV, Malaspina D, Svrakic DM, Freedman R: Further investigation of a chromosome 15 locus in schizophrenia: analysis of affected sibpairs from the NIMH Genetics Initiative. Am J Med Genet 1998; 81:308-312Crossref, Medline, Google Scholar
49. Goring HHH, Terwilliger JD, Blangero J: Large upward bias in estimation of locus-specific effects from genome-wide scans. Am J Hum Genet 2001; 69:1357Crossref, Medline, Google Scholar



