Combined Analysis of CSF Tau Levels and [123I]Iodoamphetamine SPECT in Mild Cognitive Impairment: Implications for a Novel Predictor of Alzheimer’s Disease
Abstract
OBJECTIVE: The aim of this study was to establish an objective and reliable index to predict the development of Alzheimer’s disease in a large pool of elderly patients with mild cognitive impairment. METHOD: Twenty-three patients with probable Alzheimer’s disease, 22 patients with mild cognitive impairment who eventually developed Alzheimer’s disease, eight patients with mild cognitive impairment who did not develop dementia, and 19 cognitively normal subjects were included in the study. The authors constructed a new diagnostic index, the CSF-CBF index, based on CSF tau levels divided by regional cerebral blood flow (CBF) in the posterior cingulate cortex. RESULTS: Receiver operating characteristic analysis showed that applying a cutoff value for the CSF-CBF index of 296.0 achieved a sensitivity of 88.5% and a specificity of 90.0% in discriminating mild cognitive impairment that progressed to Alzheimer’s disease from mild cognitive impairment that did not progress to Alzheimer’s disease. CONCLUSIONS: The CSF-CBF index is useful in predicting Alzheimer’s disease in subjects with mild cognitive impairment.
Although mild cognitive impairment is defined as an intermediate or transitional state from a normal cognitive state to dementia, it is likely that mild cognitive impairment may represent a complex heterogeneous condition and that some patients with mild cognitive impairment will not develop Alzheimer’s disease or other dementing disorders (1). With the promise of drugs that may delay the progression of Alzheimer’s disease, early and accurate detection of elderly patients with mild cognitive complaints who are destined to develop Alzheimer’s disease is of particular importance. In our previous studies (1, 2), we demonstrated that CSF tau levels were occasionally low and in a normal range in a subset of subjects with mild cognitive impairment who eventually developed Alzheimer’s disease, suggesting a limitation in the use of CSF tau levels alone for accurate prediction of Alzheimer’s disease. On the other hand, several groups have identified reduction of cerebral blood perfusion or cerebral glucose metabolism in the posterior cingulate cortex in very early stages of Alzheimer’s disease by using the sensitive functional neuroimaging techniques of single photon emission computed tomography (SPECT) and positron emission computed tomography (3, 4). In the current study, we constructed a new, combined index of CSF tau levels and measures of regional cerebral blood flow (CBF) by [123I]iodoamphetamine (123IMP) SPECT with the goal of predicting which subjects with mild cognitive impairment are most likely to develop Alzheimer’s disease.
Method
We examined 23 patients with probable Alzheimer’s disease (mean age=72.7 years, SD=6.5), 30 patients with mild cognitive impairment (mean age=72.0, SD=8.3), and 19 cognitively normal subjects (mean age=70.9, SD=14.3). The diagnosis of Alzheimer’s disease was made according to the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (5). Individuals with subjective memory complaints or forgetfulness who did not have objective memory deficits on standard neuropsychological tests (including the WAIS-R) were considered normal if there was no deterioration of cognitive function on follow-up for at least 2 years. The other source of normal comparison subjects was volunteers or spouses who had no impairment of cognitive performance and agreed to participate in the present study.
The diagnosis of mild cognitive impairment was made according to published criteria (6). The patients with mild cognitive impairment were divided into two separate groups: those with progressive mild cognitive impairment (N=22, mean age=71.9, SD=8.8) and those with nonprogressive mild cognitive impairment (N=8, mean age=72.1, SD=7.2). Progressive mild cognitive impairment was defined as the development of Alzheimer’s disease on follow-up (mean=3.1 years, SD=1.4). Nonprogressive mild cognitive impairment was defined as either having a transient cognitive loss under a variety of conditions or showing no progressive cognitive deterioration over time.
Baseline cognitive ability was assessed by Mini-Mental State Examination (MMSE) within 3 months of the CSF/SPECT examination. The mean MMSE score of the normal comparison subjects was 28.9 (SD=0.9); the mean score of the patients with progressive mild cognitive impairment was 25.6 (SD=1.1); the mean score of the patients with nonprogressive mild cognitive impairment was 26.6 (SD=1.3); and the mean score of the patients with Alzheimer’s disease was 17.5 (SD=3.3). After complete description of the study to the patients and comparison subjects, written informed consent was obtained from all participants.
CSF samples were obtained by lumbar puncture, and CSF tau levels were determined by enzyme-linked immunosorbent assay as described previously (1, 2). SPECT was performed with a triple-headed gamma camera (MultiSPECT3, Siemens USA, New York). 111 MBq of 123IMP, a radiotracer of cerebral blood perfusion, was infused into an antecubital vein. The regions of interest were placed in the posterior cingulate (Brodmann’s areas 23 and 31) on the sagittal images as well as in the temporoparietal region (Brodmann’s areas 39 and 40) and in the cerebellar hemisphere on the transverse images with reference to individual magnetic resonance images.
CSF samples were not obtained from one of the 22 patients with progressive mild cognitive impairment and nine of the 19 normal subjects. Four patients with progressive mild cognitive impairment, one of the eight with nonprogressive mild cognitive impairment, and five normal subjects did not undergo SPECT examination. Both CSF tau levels and SPECT images were available for all 23 patients with Alzheimer’s disease, 17 patients with progressive mild cognitive impairment, seven patients with nonprogressive mild cognitive impairment, and five normal comparison subjects.
The regional-to-cerebellar 123IMP uptake ratio (CBF ratio) was used as a measure of the relative perfusion rate in the posterior cingulate and in the temporoparietal region. The interrater reliability for the region of interest measurement was tested between two raters (N.O. and M.M.) in 32 patients. The intraclass correlation coefficient was 0.93 in the posterior cingulate and 0.81 in the temporoparietal region. The Pearson correlation coefficient between these two measurements was r=0.87 in the posterior cingulate (N=32, p<0.01) and r=0.69 in the temporoparietal region (N=32, p<0.01). A new, combined index of the CSF tau level and the CBF ratio in the posterior cingulate (CSF-CBF index) was constructed by the following equation: (CSF tau level)/(CBF ratio in the posterior cingulate).
The sensitivity and specificity of four diagnostic indexes (CSF tau level, CBF ratio in the posterior cingulate, CBF ratio in the temporoparietal region, and CSF-CBF index) to discriminate between progressive and nonprogressive mild cognitive impairment were assessed by using receiver operating characteristics analysis. Areas under the receiver operating characteristic curves were calculated by the method described by Metz et al. (7) with ROCKIT software (University of Chicago). Linear discriminant analysis also was performed to assess the diagnostic power of the CSF tau level and CBF ratio in the posterior cingulate for the discrimination between patients with progressive and nonprogressive mild cognitive impairment. Values are expressed as means and standard deviations. Statistical analysis was performed by using analysis of variance, and the significance level was defined as p<0.05.
Results
The CBF ratio in the posterior cingulate was significantly lower in the group with progressive mild cognitive impairment (mean=0.952, SD=0.081) and the group with Alzheimer’s disease (mean=0.833, SD=0.118) than the normal comparison group (mean=1.057, SD=0.075) and the group with nonprogressive mild cognitive impairment (mean=1.080, SD=0.089) (F=21.4, df=3, 58, p<0.001). The CSF tau levels in the group with progressive mild cognitive impairment (mean=566.5 pg/ml, SD=284.0) and the group with Alzheimer’s disease (mean=588.5 pg/ml, SD=255.6) were significantly higher than those in the normal comparison group (mean=230.1 pg/ml, SD=92.4) and the group with nonprogressive mild cognitive impairment (mean=254.1 pg/ml, SD=307.1) (F=7.5, df=3, 58, p<0.001). As a result, the CSF-CBF index was significantly higher in the group with progressive mild cognitive impairment (mean=644.9, SD=287.5) and the group with Alzheimer’s disease (mean=741.1, SD=408.6) than in the normal comparison group (mean=238.6, SD=130.9) and the group with nonprogressive mild cognitive impairment (mean=139.5, SD=93.7) (F=8.2, df=3, 48, p<0.001).
As shown in Figure 1, when we used a CSF-CBF index of 296.0 as a cutoff, we achieved a sensitivity of 88.5% and a specificity of 90.0% in discriminating patients with progressive mild cognitive impairment from those with nonprogressive mild cognitive impairment. The receiver operating characteristics analysis demonstrated that the area under the curve was 0.959 for the CSF-CBF index, 0.871 for the CBF ratio in the posterior cingulate, 0.850 for the CSF tau level, and 0.590 for the CBF ratio in the temporoparietal region. Finally, using a combination of CSF tau levels and the CBF ratio in the posterior cingulate, linear discriminant analysis showed a significant multivariate difference between groups with progressive and nonprogressive mild cognitive impairment (lambda=0.4, F=15.6, df=2, 21, p<0.001).
Discussion
We previously demonstrated that CSF tau levels were not convincingly higher at baseline in a subgroup of patients with mild cognitive impairment who eventually developed Alzheimer’s disease. It is possible to assume that even though tau protein is consistently released from dying brain neurons into the CSF, the CSF tau level may not always reach the pathological range if the degenerative process has begun in only a limited population of vulnerable neurons. These ideas led us to construct a unique, combined index of CSF tau levels and regional CBF measures to allow us to predict the likelihood of developing Alzheimer’s disease in a more accurate manner.
To our knowledge, histopathological changes in the posterior cingulate cortex have never been examined in the earliest stages of Alzheimer’s disease. Gomez-Isla et al. (8) reported that a substantial loss of neurons and an abundant accumulation of pathological tau isoforms were found in layers II and IV of the entorhinal cortex in brains at very early stages of Alzheimer’s disease. According to a report by Meguro et al. (9), neurotoxin-induced lesions of both entorhinal and perirhinal cortices produced a significant and long-lasting decline in cerebral glucose metabolism in the posterior cingulate cortex. The metabolic decline in the posterior cingulate cortex was reported to be significantly correlated with the severity of tissue damage in the rhinal cortex (9). Therefore, it is likely that the selective and profound hypoperfusion in the posterior cingulate cortex in the earliest stages of Alzheimer’s disease may reflect, at least in part, the entorhinal cortex damage. In fact, there is a significant correlation between posterior cingulate hypoperfusion and CSF tau levels (Maruyama et al., unpublished data).
In spite of a small number of subjects, our pilot study suggests that an abnormal CSF-CBF index in subjects with mild cognitive impairment is clinically informative in the prediction of Alzheimer’s disease. This finding may help select patients with mild cognitive impairment who are potential targets of pharmacological or nonpharmacological interventions in an attempt to delay or halt the onset of dementia.
Received April 5, 2001; revision received Aug. 30, 2001; accepted Sept. 5, 2001. From the Cyclotron and Radioisotope Center, Department of Geriatric and Respiratory Medicine and the Division of Nuclear Medicine, Tohoku University School of Medicine. Address reprint requests to Dr. Arai, Department of Geriatric and Respiratory Medicine, Tohoku University School of Medicine, Sendai, Miyagi 980-8574, Japan; [email protected] (e-mail).
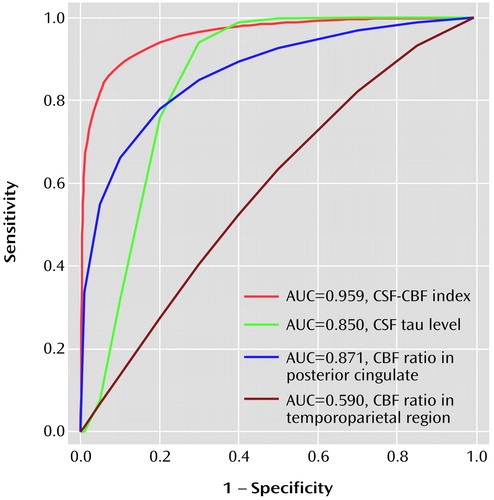
Figure 1. Receiver Operating Characteristic Curves of the Power of Four Diagnostic Indexes to Discriminate Between Patients With Mild Cognitive Impairment That Did (N=22) or Did Not (N=8) Progress to Alzheimer’s Diseasea
aAUC is the area under the curve. CBF (cerebral blood flow) ratio is the ratio of regional to cerebellar [123I]iodoamphetamine uptake according to single photon emission computed tomography. The CSF-CBF index yielded the most appropriate sensitivity and specificity. CSF sample was not available for one patient with progressive cognitive impairment. CBF data were not available for four patients with progressive impairment and one patient with nonprogressive impairment.
1. Arai H, Nakagawa T, Kosaka T, Higuchi M, Matsui T, Okamura N, Tashiro M, Sasaki H: Elevated cerebrospinal fluid tau protein level as a predictor of dementia in memory-impaired individuals. Alzheimer’s Res 1997; 3:211-213Google Scholar
2. Arai H, Ishiguro K, Ohno H, Moriyama M, Itoh N, Okamura N, Matsui T, Morikawa Y, Horikawa E, Kohno H, Sasaki H, Imahori K: CSF phosphorylated tau protein and mild cognitive impairment: a prospective study. Exp Neurol 2000; 166:201-203Crossref, Medline, Google Scholar
3. Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE: Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol 1997; 42:85-94Crossref, Medline, Google Scholar
4. Johnson KA, Jones K, Holman BL, Becker JA, Spiers PA, Satlin A, Albert MS: Preclinical prediction of Alzheimer’s disease using SPECT. Neurology 1998; 50:1563-1571Crossref, Medline, Google Scholar
5. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM: Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34:939-944Crossref, Medline, Google Scholar
6. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E: Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999; 56:303-308Crossref, Medline, Google Scholar
7. Metz CE, Herman BA, Roe CA: Statistical comparison of two ROC-curve estimates obtained from partially-paired datasets. Med Decis Making 1998; 18:110-121Crossref, Medline, Google Scholar
8. Gomez-Isla T, Price JL, McKeel DW Jr, Morris JC, Growdon JH, Hyman BT: Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci 1996; 16:4491-4500Crossref, Medline, Google Scholar
9. Meguro K, Blaizot X, Kondoh Y, Le Mestric C, Baron JC, Chavoix C: Neocortical and hippocampal glucose hypometabolism following neurotoxic lesions of the entorhinal and perirhinal cortices in the non-human primate as shown by PET: implications for Alzheimer’s disease. Brain 1999; 122:1519-1531Crossref, Medline, Google Scholar



