Depressive Symptoms and Severity of Illness in Multiple Sclerosis: Epidemiologic Study of a Large Community Sample
Abstract
OBJECTIVE: Previous research has shown high prevalence rates of depression in multiple sclerosis patients seen in specialty clinics. The relationships among depressive symptoms and severity, duration, and course of multiple sclerosis are controversial. METHOD: A survey was mailed to members of the Multiple Sclerosis Association of King County (Wash.). Of the 1,374 eligible participants, 739 returned the survey, a response rate of 53.8%. Data about demographic characteristics, employment, and duration and course of multiple sclerosis were collected. Severity of multiple sclerosis was determined by the Expanded Disability Status Scale, self-report version. Severity of depressive symptoms was evaluated with the Center for Epidemiologic Studies Depression Scale (CES-D Scale). Analysis of covariance was used to compare mean CES-D Scale scores across categories of multiple sclerosis, and logistic regression was used to identify variables associated with clinically significant depression. RESULTS: Clinically significant depressive symptoms (CES-D Scale score ≥16) were found in 41.8% of the subjects, and 29.1% of the subjects had moderate to severe depression (score ≥21). Subjects with advanced multiple sclerosis were much more likely to experience clinically significant depressive symptoms than subjects with minimal disease. Shorter duration of multiple sclerosis was associated with a greater likelihood of significant depressive symptoms, but the pattern of illness progression was not. CONCLUSIONS: In this large community sample, the severity of multiple sclerosis was more strongly associated with depressive symptoms than was pattern of illness. Clinicians should evaluate depression in patients with recent diagnoses of multiple sclerosis, major changes in functioning, or limited social support.
Multiple sclerosis is a demyelinating disease of the CNS that affects 1 in 1,000 people in Western countries (1). It is the most common chronic disabling CNS disease in young adults. A diagnosis of multiple sclerosis requires the occurrence of at least two neurological events consistent with demyelination in the CNS that are separated temporally and anatomically. Early onset (typically between 20 and 40 years of age) and long duration of disease result in tremendous individual, family, and societal costs as well as reductions in quality of life and work productivity (2). Multiple sclerosis has a variable and unpredictable course, with symptoms that can include weakness, visual loss, bowel and bladder incontinence, fatigue, cognitive impairment, and mood symptoms.
Depression may be more common in multiple sclerosis than in other chronic neurological conditions (3, 4). Epidemiologic studies of patients at specialty clinics (5–8) have indicated that the lifetime risk of major depression in multiple sclerosis is between 22.8% and 54.0%. Small studies (9, 10) also suggest a wide range for the point prevalence of depression in multiple sclerosis, 27%–54%. These studies have used a variety of screening measures to detect depression and have been conducted in specialty clinics, which may not be representative of the underlying population. Risk factors for major depression in multiple sclerosis were identified in one study (5) and included female gender, age less than 35 years, family history of major depression, and a high level of stress.
Higher levels of disability have been associated with more severe depressive symptoms in several chronic illnesses (11). While the relationship between depression and functional disability in multiple sclerosis has been widely investigated, the relationship remains controversial (12). Some studies (13, 14) suggest that patients with greater disability are more likely to experience depression. Other authors (15) claim that the frequency or severity of depressive episodes among multiple sclerosis patients is independent of the severity of multiple sclerosis, as reflected by the patient’s score on the Expanded Disability Status Scale (16). Stenager et al. (17) noted a tendency for depressive symptoms to increase with worsening disability up to moderate disability (score of 5.0 or 6.0 on the Expanded Disability Status Scale). Neither duration of illness nor the occurrence of relapses has been found to be associated with depression in most studies (18).
In this cross-sectional study we examined the prevalence of clinically significant depressive symptoms in a large community sample of patients with multiple sclerosis by using the Center for Epidemiological Study Scale for Depression (CES-D Scale) (19). This instrument has been validated and widely used in medical settings to screen for depression. We evaluated whether there are different prevalence rates of depressive symptoms across categories of multiple sclerosis: severity of illness, duration of illness, and course of illness. We also examined the association of depressive symptoms with decrements in specific areas of functioning, such as vision, ambulation, and cognitive functioning.
Method
A mail survey was sent to 1,453 members of the Multiple Sclerosis Association of King County (Wash.). A reminder card and two additional mailings of the survey were sent to increase the response rate (20). Information on nonresponders was not available, as the surveys were mailed by the association, and study investigators did not have access to the mailing list. The association reported that five surveys were returned because of incorrect addresses. There were 19 duplicate addresses, 16 addressees were deceased, and 39 reported that they did not have multiple sclerosis. Of the 1,374 possible participants, 739 returned the survey, for a response rate of 53.8%. The Human Subjects Committee at the University of Washington approved the study protocol.
The survey was designed to assess multiple dimensions of multiple sclerosis, as well as depressive symptoms and social support. The survey included questions on demographic characteristics, such as marital status, employment status, and educational level. The patients were asked about the date of their multiple sclerosis diagnosis and whether the diagnosis had been confirmed by magnetic resonance imaging (MRI), lumbar puncture, or evoked potential studies. Each patient was also asked to indicate his or her course of illness by choosing one of five diagrams. Course of illness was then categorized into relapsing-remitting, primary progressive, and secondary progressive.
Depressive symptoms were measured by using the CES-D Scale. This 20-item self-rating scale was developed to screen for depression in primary care settings, and it has been shown to be reliable and valid when used for this purpose (21). Both Radloff (19) and Weissman et al. (22) have recommended using a cutoff score of 16 to define a clinically significant level of depressive symptoms. Following Unutzer et al. (23), we defined four groups of depressive symptoms: 1) little or no symptoms of depression (CES-D Scale score <16), 2) mild depressive symptoms (score=16–20), 3) moderate depressive symptoms (score=21–25) (24), and 4) severe depression (score=26–60).
The Expanded Disability Status Scale (16) is the standard measure of disease progression and the degree of neurological impairment in clinical practice and clinical trials. For this mail survey, we used a self-report version of the Expanded Disability Status Scale, which had been designed and validated in a previous study (25). Results with this patient-administered scale are strongly correlated with those from the physician-administered scale, and the interrater variability is similar to that seen between two physician-administered tests (25). The Expanded Disability Status Scale divides functioning into eight functional systems: pyramidal, cerebellar, brainstem, cerebral, bowel and bladder, sensory, visual, and other; impairment in each system is graded and then summed across the eight systems. Scores for the total scale can range from 0 (no neurological abnormality) to 10 (death from multiple sclerosis). In our analyses, the scores were categorized as indicating minimal (0.0–4.0), intermediate (4.5–6.5), and advanced (7.0–9.5) severity of multiple sclerosis to reflect milestones in progressive loss of functioning. A patient with a score of 4.0 or lower is ambulatory (able to walk without aid or rest for more than 500 m) and independent; a patient with a score in the intermediate range likely has disability severe enough to limit daily activities; a patient with a score of 7.0 is essentially restricted to a wheelchair.
Social support was measured by using the five-item version of the Modified Social Support Survey (26). The Modified Social Support Survey is an adaptation of the Social Support Survey, a brief multidimensional measure of perceived social support developed in the Medical Outcomes Study, and is a part of the Multiple Sclerosis Quality of Life Inventory (27).
Logistic regression modeling was used to identify factors significantly associated with a CES-D Scale score of 16 above. Age, gender, marital status, race, course of illness, disability level, duration of illness, and social support were candidates for the multivariate model. All variables significant at the 0.05 level according to a two-sided Wald test (chi-square) were included. Because this was a cross-sectional study, prevalence odds ratios were calculated, with 95% confidence intervals (CIs). The model fit was tenable, and there were no outliers of concern. Plausible interactions were assessed, but none was significant.
Mean CES-D Scale scores for the three severity levels were adjusted for age, gender, education, and marital status by using analysis of covariance. The significance of the severity variable was assessed with an F test, with the critical level alpha=0.05. The Bonferroni correction was used to compensate for multiple comparisons of the least-squared means for each level. Means for the course and duration of multiple sclerosis were computed in a similar manner, with the addition of an adjustment for severity of disease.
Results
The study sample had a mean age of 49.3 years (SD=11.3, range=21–83) and a mean duration of multiple sclerosis of 12.5 years (SD=9.5, range=0–52). Nearly all (91.9%) of the subjects reported that the diagnosis of multiple sclerosis had been confirmed by MRI, lumbar puncture, or evoked potentials. Other demographic and clinical characteristics of the study sample are given in Table 1; reflecting the population of patients with multiple sclerosis (28), the respondents were predominantly Caucasian and female. The educational level of the sample is consistent with findings from a large national database of persons with multiple sclerosis (29), in which only 4.3% had fewer than 12 years of education and 52.6% had some college or higher education. The relative frequencies of the courses of multiple sclerosis were consistent with the findings of previous epidemiologic studies (30); half of the sample had a relapsing-remitting course. The scores on the Expanded Disability Status Scale reflected a range of severity of illness; nearly one-fourth of the sample had minimal severity of multiple sclerosis, and nearly one-third were in the advanced severity category.
The mean CES-D Scale score for the study sample was 16.0 (SD=11.1, range=0–54). Large epidemiologic studies suggest that a cutoff score of 16 is consistent with clinically significant depressive symptoms. According to this standard, 41.8% of the study sample had clinically significant depressive symptoms. Studies in primary care settings of patients with higher levels of comorbid medical illness have suggested that a cutoff of 21 has the best positive predictive value for major depression (31). According to this standard, 29.1% of the sample had clinically significant depressive symptoms. Approximately equal proportions of depressed subjects had mild, moderate, and severe depressive symptoms (Figure 1, first bar).
Logistic regression modeling was used to determine which factors were significantly associated with clinically significant depressive symptoms (CES-D Scale score ≥16) (Table 2). Severity of illness, as reflected by the score on the Expanded Disability Status Scale, was the most significant factor. The odds of depressive symptoms in subjects with intermediate illness was three times that of the group with minimal severity of multiple sclerosis, and the odds for those with advanced severity was six times as high. Consistent with this, the mean adjusted CES-D Scale scores were higher with increasing severity of multiple sclerosis (Table 3). The group with minimal severity was not, on average, depressed (that is, they had a mean CES-D Scale score below 16). The mean CES-D Scale scores for the two other severity categories were clinically comparable. The difference was also not statistically significant (t=0.77, df=538, p=0.45). The severity of depressive symptoms was also different across categories of severity of multiple sclerosis; approximately 20% of the patients with intermediate or advanced multiple sclerosis were severely depressed (Figure 1).
An additional analysis was performed, and it showed that the differences in CES-D Scale scores among categories of the Expanded Disability Status Scale were not due solely to symptoms that might be better explained by multiple sclerosis. Four items from the CES-D Scale (“I had trouble keeping my mind on what I was doing,” “I felt that everything I did was an effort,” “My sleep was restless,” and “I could not get going”) were deleted. The group with minimal illness severity retained significantly lower mean scores than patients with more severe multiple sclerosis; their mean score was 8.1 (SD=10.1), whereas the scores for those with intermediate and advanced multiple sclerosis were 12.0 (SD=10.0) and 13.2 (SD=9.7), respectively (F=13.22, df=2, 699, p<0.0001).
Adjusted for severity of illness, course of illness (relapsing-remitting, primary progressive, or secondary progressive) was not associated with CES-D Scale score. The mean scores were clinically similar for the three courses, and there were no statistically significant differences among these means (Table 3).
Subjects with a shorter duration of multiple sclerosis were more likely to have clinically significant depressive symptoms (Table 2), suggesting the occurrence of adjustment to illness over time. A duration 10 years shorter than the mean was associated with a 36% greater risk of depression (CES-D Scale score ≥16). The 28 subjects who were within 1 year of diagnosis had a much higher mean CES-D Scale score (Table 3), but the mean values for the three duration categories were not statistically significantly different. Severe depressive symptoms (CES-D score=26–60) were present in a larger proportion of subjects who had received diagnoses within the last year (32.1%) than in those given diagnoses 1–5 years (21.8%) or more than 5 years (10.3%) earlier, but the differences between these percentages were also not statistically significant. The mean CES-D Scale score increased with increasing severity of illness, regardless of duration of multiple sclerosis (Figure 2).
Other factors significantly associated with a CES-D Scale score of 16 or higher in logistic regression modeling were lower education level, younger age, and lack of social support (Table 2). It is interesting that gender was not associated with depression in this sample. Analysis of the functional systems of the Expanded Disability Status Scale revealed that all were significantly univariately associated with depressive symptoms, even after controls for age, gender, educational level, and duration of multiple sclerosis. Self-report of cognitive difficulties was the most strongly associated with clinically significant depressive symptoms (prevalence odds ratio=1.87, 95% CI=1.63–2.14, Wald test χ2=72.9, df=1, p<0.0001).
Discussion
Of the subjects in this large community sample of persons with multiple sclerosis, 41.8% had clinically significant depressive symptoms. More than one-fourth (29.1%) had a CES-D Scale score of 21 or higher, which has been shown to predict major depression (31). These numbers suggest that there is a high prevalence of depressive symptoms among community-dwelling persons with multiple sclerosis, not just multiple sclerosis patients seen in specialty clinics. Moreover, it appears that most persons with multiple sclerosis who report depressive symptoms are likely to have clinically significant depressive symptoms, as 69.6% of the subjects with a CES-D Scale score of 16 or higher had a CES-D Scale score of 21 or higher.
These rates are much higher than rates of depressive symptoms found in studies that used a CES-D Scale score of 16 or higher to screen for depression in the general population (point prevalence of 3%–9%) (32) or primary care populations (point prevalence of 10%–15%) (33). These rates are also higher than those found in studies of persons with other chronic medical illnesses. Lyketsos et al. (34) reported that 21.3% of a large sample of men with HIV disease had a CES-D Scale score of 16 or higher. The results of this study are consistent with findings of depression in neurologic disorders, such as Parkinson’s disease. Several studies that used more stringent diagnostic criteria for depression (structured psychiatric interviews) (35–37) indicated prevalences of depression in Parkinson’s disease of 41%–49%. It is interesting that at least one study (38) has suggested that persons with multiple sclerosis that predominantly affects the spinal cord and cerebellum have a lower rate of depression (as measured by structured psychiatric interview) than do persons with predominantly cerebral involvement.
In this large community sample of persons with multiple sclerosis, severity of multiple sclerosis was more strongly associated with depressive symptoms than was duration of illness or pattern of progression. Subjects with intermediate and advanced illness according to the Expanded Disability Status Scale were three and six times, respectively, as likely as subjects with minimal disease to have clinically significant depressive symptoms. These findings are consistent with longitudinal findings on chronic medical illness and normal aging that suggest that depressive symptoms and major depressive disorder develop as functional impairment increases (39). However, as this study was cross-sectional, we cannot conclude that increased disability or illness severity causes depression.
Depression is associated with greater disability and amplification of somatic symptoms in other medical illnesses (40). The Expanded Disability Status Scale score is based largely on objective measurement of physical mobility and may be less affected by depression than self-reports of functional impairment or symptoms (such as pain). Of the eight functional systems in the Expanded Disability Status Scale, cognitive symptoms had the strongest association with depressive symptoms, a relationship that has been suggested by previous studies (41). The strong association between Expanded Disability Status Scale score and depressive symptoms was not entirely due to the self-report of cognitive symptoms, however. In a logistic regression analysis controlling for the cognitive symptom subscale, as well as the other variables in Table 2, there were still strong associations between illness severity category and depressive symptoms. The odds ratio for clinically significant depressive symptoms in subjects with intermediate severity of multiple sclerosis was 2.01 (95% CI=1.18–3.44, χ2=6.5, df=1, p=0.02); the odds ratio for subjects with advanced multiple sclerosis was 3.85 (95% CI=2.03–7.30, χ2=17.0, df=1, p<0.0001).
A recent large observational study of the natural history of multiple sclerosis (42) indicated that relapses do not significantly influence the progression of irreversible disability. Our study suggests that a relapsing-remitting course also may not be the most important factor in the development of depressive symptoms. The lower prevalence of severe depressive symptoms in patients with longer durations of multiple sclerosis suggests that patients may adapt to illness over time. Subjects who were within 1 year of the diagnosis of multiple sclerosis were more often severely depressed. While with only 28 recently diagnosed subjects, this difference was not statistically significant, the finding is consistent with previous findings about initial adjustment to illnesses such as cancer (43).
Younger age, less education, and lack of social support were also associated with depressive symptoms in this sample, as they are in the general population and in samples of patients with other medical illnesses (44). The lack of association between CES-D Scale score and female gender was a surprising finding, as depression is 1.7 to 2.0 times as common in women in the general population (45). This may reflect a sampling bias in our study: the male subjects had a higher mean score on the Expanded Disability Status Scale (6.10 compared to 5.68 for women) (t=–2.72, df=1, p=0.02). However, even after we controlled for severity of multiple sclerosis, gender was not associated with a CES-D Scale score of 16 or higher. Some small studies have suggested that in multiple sclerosis depression may not be associated with gender (18) and that hormonal or biological factors may, in part, explain this lack of association. These findings need to be replicated by other studies, which should include information about past history and current treatment.
This cross-sectional mail survey had several limitations. First, symptoms and limitations were self-reported. Bowen et al. (25) have shown that the self-administered Expanded Disability Status Scale performs well, but direct physical examinations or clinical evaluations were not performed on subjects in this study. Second, persons with multiple sclerosis have somatic complaints (such as fatigue and cognitive complaints) that are not easily distinguishable from depressive symptoms, and screening instruments might overrepresent the level of depression in the sample (46). However, when a sensitivity analysis was performed to compare mean CES-D Scale scores across the categories of the Expanded Disability Status Scale after the four somatic items were removed from the instrument, the mean CES-D Scale scores still increased significantly with increasing severity of multiple sclerosis. Third, data were not available from persons who did not respond to the survey, raising the concern that persons with depressive symptoms were either over- or underrepresented in the sample. However, the demographic and disease-specific characteristics of this sample are similar to those of the entire Multiple Sclerosis Association of King County (personal communication from Merrill Ringgold, Executive Director), suggesting that the sample is representative of community-dwelling persons with multiple sclerosis. Finally, as the study is cross-sectional, we cannot imply causation about these interesting associations. Prospective studies and treatment trials are needed to examine the relationships among depression, disability, and illness severity.
These findings have several clinical implications. First, depressive symptoms are common in multiple sclerosis and should be screened for and evaluated routinely by multiple sclerosis clinicians in the same way as other aspects of functioning (such as ambulation and vision). Screens such as the CES-D Scale are easy to administer, are useful as an initial screen (47), and can detect clinical change across time (48). If substantial depressive symptoms are detected, referral to a mental health provider can be made for a more complete evaluation. Aggressive treatment with pharmacotherapy or psychotherapy should be considered. Although few randomized, controlled trials have been conducted in this population, a meta-analysis (49) indicated that both pharmacotherapy and psychotherapy are effective. Studies have shown that depression negatively affects physical, cognitive, social, and work functioning in other medical populations (50). Treatment of depression has the potential not only to reduce suffering but also to minimize this additional disability.
Clinicians should be particularly alert to the possibility of depression in patients with recent diagnoses of multiple sclerosis, at times of major change or loss of functioning, and in patients with limited social support. Younger persons with multiple sclerosis may be more likely to be depressed, and men may be as likely as women to be affected. Many questions remain about the relative contributions of biological factors, personal characteristics, and social environments to depression in multiple sclerosis. This study suggests that the rate of depressive symptoms in patients with multiple sclerosis is similar to rates in patients with other CNS disorders and is higher than in patients with other chronic medical illnesses. The explanation likely involves multiple factors, including direct effects of multiple sclerosis on limbic system structures and the negative effects of functional impairment on life satisfaction and self-esteem.
 |
 |
 |
Received May 18, 2001; revisions received Oct. 29, 2001, and April 19 and June 7, 2002; accepted June 11, 2002. From the Multiple Sclerosis Rehabilitation Research and Training Center, University of Washington, Seattle. Address reprint requests to Dr. Chwastiak, Department of Psychiatry and Behavioral Sciences, Harborview Medical Center, Box 359911, Seattle, WA 98104; [email protected] (e-mail). Supported by grant H133B980017 from the National Institute of Disability and Rehabilitation Research, U.S. Department of Education. The authors thank the Multiple Sclerosis Association of King County for its work in conducting the survey, Dr. Wayne Katon for reviewing drafts of the manuscript, and Carolyne Dollar for assistance in preparing the manuscript.
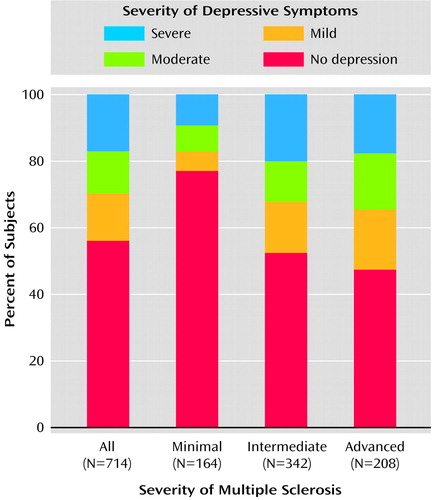
Figure 1. Severity of Depressive Symptoms in a Community Sample of Patients With Multiple Sclerosis, by Severity of Multiple Sclerosisa
aSeverity of depressive symptoms was determined with the Center for Epidemiologic Studies Depression Scale (no depression, score <16; mild, score=16–20; moderate, score=21–25; severe, score=26–60). Severity of multiple sclerosis was determined with the Expanded Disability Status Scale (16) (minimal, score=0.0–4.0; intermediate, score=4.5–6.5; advanced, score=7.0–9.5).
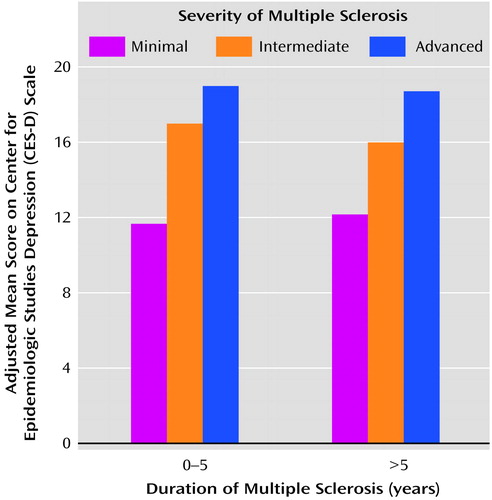
Figure 2. Depression Scores for a Community Sample of 714 Patients With Minimally Severe, Intermediate, or Advanced Multiple Sclerosis, by Time Since Diagnosisa
aCES-D scores were adjusted for age, gender, education, and marital status. Severity of multiple sclerosis was determined with the Expanded Disability Status Scale (16) (minimal, score=0.0–4.0; intermediate, score=4.5–6.5; advanced, score=7.0–9.5).
1. Sadovnick AD, Ebers GC: Epidemiology of multiple sclerosis: a critical overview. Can J Neurol Sci 1993; 20:17-29Crossref, Medline, Google Scholar
2. Grima DT, Torrance GW, Francis G, Rice G, Rosner AJ, Lafortune L: Cost and health related quality of life consequences of multiple sclerosis. Mult Scler 2000; 6:91-98Crossref, Medline, Google Scholar
3. Schiffer RB, Babigian HM: Behavioral disorders in multiple sclerosis, temporal lobe epilepsy, and amyotrophic lateral sclerosis: an epidemiologic study. Arch Neurol 1984; 41:1067-1069Crossref, Medline, Google Scholar
4. Schubert DS, Foliart RH: Increased depression in multiple sclerosis patients: a meta-analysis. Psychosomatics 1993; 34:124-130Crossref, Medline, Google Scholar
5. Patten SB, Metz LM, Reimer MA: Biopsychosocial correlates of lifetime major depression in a multiple sclerosis population. Mult Scler 2000; 6:115-120Crossref, Medline, Google Scholar
6. Sadovnick AD, Remick RA, Allen J, Swartz E, Yee IM, Eisen K, Farquhar R, Hashimoto SA, Hooge J, Kastrukoff LF, Morrison W, Nelson J, Oger J, Paty DW: Depression and multiple sclerosis. Neurology 1996; 46:628-632Crossref, Medline, Google Scholar
7. Minden SL, Orav J, Reich P: Depression in multiple sclerosis. Gen Hosp Psychiatry 1987; 9:426-434Crossref, Medline, Google Scholar
8. Joffe RT, Lippert GP, Gray TA, Sawa G, Horvath Z: Mood disorder and multiple sclerosis. Arch Neurol 1987; 44:376-378Crossref, Medline, Google Scholar
9. Whitlock FA, Siskind MM: Depression as a major symptom of multiple sclerosis. J Neurol Neurosurg Psychiatry 1980; 43:861-865Crossref, Medline, Google Scholar
10. Dalos NP, Rabins PV, Brooks BR, O’Donnell P: Disease activity and emotional state in multiple sclerosis. Ann Neurol 1983; 13:573-577Crossref, Medline, Google Scholar
11. Schubert DS, Taylor C, Lee S, Mentari A, Tamaklo W: Physical consequences of depression in the stroke patient. Gen Hosp Psychiatry 1992; 14:69-76Crossref, Medline, Google Scholar
12. Millefiorini E, Padovani A, Pozzilli C, Loriedo C, Bastianello S, Buttinelli C, Di Piero V, Fieschi C: Depression in the early phase of MS: influence of functional disability, cognitive impairment and brain abnormalities. Acta Neurol Scand 1992; 86:354-358Crossref, Medline, Google Scholar
13. Surridge D: An investigation into some psychiatric aspects of multiple sclerosis. Br J Psychiatry 1969; 115:749-764Crossref, Medline, Google Scholar
14. McIvor GP, Riklan M, Reznikoff M: Depression in multiple sclerosis as a function of length and severity of illness, age, remissions, and perceived social support. J Clin Psychol 1984; 40:1028-1033Crossref, Medline, Google Scholar
15. Rabins PV, Brooks BR, O’Donnell P, Pearlson GD, Moberg P, Jubelt B, Coyle P, Dalos N, Folstein MF: Structural brain correlates of emotional disorder in multiple sclerosis. Brain 1986; 109(part 4):585-597Google Scholar
16. Kurtzke JF: Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33:1444-1452Crossref, Medline, Google Scholar
17. Stenager E, Knudsen L, Jensen K: Correlation of Beck Depression Inventory score, emotional disturbances, and duration of multiple sclerosis, in Mental Disorders and Cognitive Deficits in Multiple Sclerosis. Edited by Jensen K, Knudsen L, Stenager E, Grant I. London, John Libbey, 1989, pp 1138-1141Google Scholar
18. Moller A, Wiedemann G, Rohde U, Backmund H, Sonntag A: Correlates of cognitive impairment and depressive mood disorder in multiple sclerosis. Acta Psychiatr Scand 1994; 89:117-121Crossref, Medline, Google Scholar
19. Radloff LS: The CES-D Scale: a self-report depression scale for research in the general population. J Applied Psychol Measurement 1977; 1:385-401Crossref, Google Scholar
20. Salant P, Dillman D: How to Conduct Your Own Survey. New York, John Wiley & Sons, 1994Google Scholar
21. Myers JK, Weissman MM: Use of a self-report symptom scale to detect depression in a community sample. Am J Psychiatry 1980; 137:1081-1084Link, Google Scholar
22. Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ: Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol 1977; 106:203-214Crossref, Medline, Google Scholar
23. Unutzer J, Patrick DL, Marmon T, Simon GE, Katon WJ: Depressive symptoms and mortality in a prospective study of 2,558 older adults. Am J Geriatr Psychiatry 2002; 10:521-530Crossref, Medline, Google Scholar
24. Lyness JM, Duberstein PR, King DA, Cox C, Caine ED: Medical illness burden, trait neuroticism, and depression in older primary care patients. Am J Psychiatry 1998; 155:969-971Link, Google Scholar
25. Bowen J, Gibbons L, Gianas A, Kraft G: Self-administered Expanded Disability Status Scale with functional system scores correlates well with physician-administered test. Mult Scler 2001; 7:201-206Crossref, Medline, Google Scholar
26. Sherbourne CD, Stewart AL: The MOS social support survey. Soc Sci Med 1991; 32:705-714Crossref, Medline, Google Scholar
27. Ritvo PG, Fischer JS, Miller DM, Andrews H, Paty DW, LaRocca NG: Multiple Sclerosis Quality of Life Inventory (MSQLI): A User’s Manual. New York, National Multiple Sclerosis Society, 1997Google Scholar
28. Hogancamp WE, Rodriguez M, Weinshenker BG: The epidemiology of multiple sclerosis. Mayo Clin Proc 1997; 72:871-878Crossref, Medline, Google Scholar
29. Minden S, Marder WD, Harrold LN, Dor A: Multiple Sclerosis: A Statistical Portrait. Cambridge, Mass, Abt Associates, 1993Google Scholar
30. Wingerchuk DM, Weinshenker BG: Multiple sclerosis: epidemiology, genetics, classification, natural history and clinical outcome measures. Neuroimaging Clin North Am 2000; 10:611-624Medline, Google Scholar
31. Schulberg HC, Saul M, McClelland M, Ganguli M, Christy W, Frank R: Assessing depression in primary medical and psychiatric practices. Arch Gen Psychiatry 1985; 42:1164-1170Crossref, Medline, Google Scholar
32. Eaton WW, Kessler LG: Rates of symptoms of depression in a national sample. Am J Epidemiol 1981; 114:528-538Crossref, Medline, Google Scholar
33. Coyne JC, Fechner-Bates S, Schwenk TL: Prevalence, nature and comorbidity of depressive disorders in primary care. Gen Hosp Psychiatry 1994; 16:267-276Crossref, Medline, Google Scholar
34. Lyketsos CG, Hoover DR, Guccione M, Senterfitt W, Dew MA, Wesch J, VanRaden MJ, Tresiman GJ, Morgenstern H: Depressive symptoms as predictors of medical outcomes in HIV infection: Multicenter AIDS Cohort Study. JAMA 1993; 270:2563-2567Crossref, Medline, Google Scholar
35. Mayeux R, Stern Y, Williams JBW, Cote L, Frantz A, Dyrenfurth I: Clinical and biochemical features of depression in Parkinson’s disease. Am J Psychiatry 1986; 143:756-759Link, Google Scholar
36. Dooneief G, Mirabello E, Bell K, Marder K, Stern Y, Mayeux R: An estimate of the incidence of depression in idiopathic Parkinson’s disease. Arch Neurol 1992; 49:305-307Crossref, Medline, Google Scholar
37. Gotham AM, Brown RG, Marsden CD: Depression in Parkinson’s disease: a quantitative and qualitative analysis. J Neurol Neurosurg Psychiatry 1986; 49:381-389Crossref, Medline, Google Scholar
38. Schiffer RB, Caine ED, Bamford KA, Levy S: Depressive episodes in patients with multiple sclerosis. Am J Psychiatry 1983; 140:1498-1500Link, Google Scholar
39. Ormel J, VonKorff M, Oldehinkel AJ, Simon G, Tiemens BG, Ustun TB: Onset of disability in depressed and non-depressed primary care patients. Psychol Med 1999; 29:847-853Crossref, Medline, Google Scholar
40. Katon W, Sullivan M, Walker E: Medical symptoms without identified pathology: relationship to psychiatric disorders, childhood and adult trauma, and personality traits. Ann Intern Med 2001; 134:917-925Crossref, Medline, Google Scholar
41. Cutajar R, Ferriani E, Scandellari C, Sabattini L, Trocino C, Marchello LP, Stecchi S: Cognitive function and quality of life in multiple sclerosis patients. J Neurovirol 2000; 6(suppl 2):S186-S190Google Scholar
42. Confavreux C, Vukusic S, Moreau T, Adeleine P: Relapses and progression of disability in multiple sclerosis. N Engl J Med 2000; 343:1430-1438Crossref, Medline, Google Scholar
43. Grassi L, Malacarne P, Maestri A, Ramelli E: Depression, psychosocial variables and occurrence of life events among patients with cancer. J Affect Disord 1997; 44:21-30Crossref, Medline, Google Scholar
44. Eaton WW, Kramer M, Anthony JC, Dryman A, Shapiro S, Locke BZ: The incidence of specific DIS/DSM-III mental disorders: data from the NIMH Epidemiologic Catchment Area Program. Acta Psychiatr Scand 1989; 79:163-178Crossref, Medline, Google Scholar
45. Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB: Sex and depression in the National Comorbidity Survey, I: lifetime prevalence, chronicity and recurrence. J Affect Disord 1993; 29:85-96Crossref, Medline, Google Scholar
46. Nyenhuis DL, Rao SM, Zajecka JM, Luchetta T, Bernardin L, Garron DC: Mood disturbance versus other symptoms of depression in multiple sclerosis. J Int Neuropsychol Soc 1995; 1:291-296Crossref, Medline, Google Scholar
47. Roberts RE, Vernon SW: The Center for Epidemiologic Studies Depression Scale: its use in a community sample. Am J Psychiatry 1983; 140:41-46Link, Google Scholar
48. Klinkman MS, Schwenk TL, Coyne JC: Depression in primary care—more like asthma than appendicitis: the Michigan Depression Project. Can J Psychiatry 1997; 42:966-973Crossref, Medline, Google Scholar
49. Mohr DC, Goodkin DE: Treatment of depression in multiple sclerosis: review and meta-analysis. Clin Psychol—Science and Practice 1999; 6:1-9Crossref, Google Scholar
50. Katon W: The impact of major depression on chronic medical illness. Gen Hosp Psychiatry 1996; 18:215-219Crossref, Medline, Google Scholar



