Sensory Gating Deficit Expressed by a Disturbed Suppression of the P50 Event-Related Potential in Patients With Alzheimer’s Disease
Abstract
OBJECTIVE: Disturbed sensory gating has been related to attention deficit and greater distractibility in patients with schizophrenia, and dysfunction of the alpha-7 subunit of the cholinergic nicotinic receptor has been discussed as its biological basis. Alzheimer’s disease is characterized by a cholinergic deficit, and postmortem studies have reported alpha-7 receptor loss in patients with Alzheimer’s disease. In this study, the authors tested whether sensory gating is disturbed in patients with Alzheimer’s disease. METHOD: Suppression of the P50 event-related potential following the second click of a double-click paradigm, a measure of sensory gating, was assessed in 17 Alzheimer’s disease patients and 17 comparison subjects. RESULTS: Alzheimer’s disease patients showed less P50 suppression following the second click relative to the comparison subjects. CONCLUSIONS: Disturbed sensory gating might result from cholinergic dysfunction and possibly from alpha-7 nicotinic receptor loss in patients with Alzheimer’s disease. Prospective studies should investigate the relationship between sensory gating deficit and behavioral disturbances in Alzheimer’s disease patients.
Sensory gating refers to the inhibition of a stimulus-related neuronal response if the stimulus is preceded by a warning tone. It can be assessed by the amplitude reduction of the P50 event-related potential to the second click of a double-click paradigm. Disturbed P50 suppression has been related to impairment in sustained attention (1) and greater distractibility (2) in patients with schizophrenia. Animal experiments (3) and postmortem studies (4) of schizophrenic patients have suggested that sensory gating is mediated by the alpha-7 subunit of the cholinergic nicotinic receptor.
Alzheimer’s disease is characterized by a presynaptic cholinergic deficit, and there is evidence for alpha-7 receptor loss (5). Beside cognitive dysfunction, behavioral symptoms related to attention deficit and greater distractibility occur in the course of Alzheimer’s disease. In this study, we investigated whether Alzheimer’s disease patients display disturbed sensory gating expressed by a deficit in P50 suppression.
Method
Seventeen patients with Alzheimer’s disease (11 women and six men; mean age=71.2 years, SD=5.8; Mini-Mental State Examination [MMSE] score=17.5, SD=5.4) and 17 healthy comparison subjects (11 women and six men; mean age=67.8 years, SD=7.4; mean MMSE score=29.1, SD=1.0) participated in the study. Presence of Alzheimer’s disease was confirmed per the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association. After complete description of the protocol, written informed consent was obtained from the subjects and their legal guardians.
The P50 suppression paradigm was performed in three blocks of 36 click pairs (volume=80 dB, stimulus duration=1 msec, interclick interval=500 msec, interpair interval=10 seconds) administered through headphones. Electrodes (resistance <10 kΩ) were placed at the Fz, Cz, and Pz recording sites with linked mastoids as the reference. EEG responses were band-pass filtered (0.53 to 500 Hz at a sampling rate of 1,024 Hz). Ocular movements were controlled with vertical and horizontal electro-oculography (EOG). After eye movement correction (6) and artifact exclusion (7), the EEG data between 100 msec before the first click and 500 msec after the second click were averaged over the three blocks. The resulting event-related potential waveforms were digitally band-pass filtered (10–50 Hz) to remove N100 effects, which can cover the P50 (8).
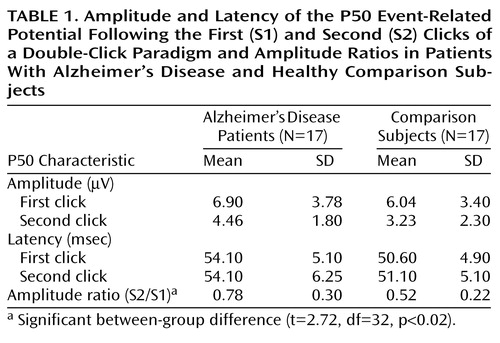
The first P50 component was defined as the most positive peak between 40–80 msec after the first click (S1). The second P50 component was defined as the positive peak after the second click (S2) that was closest to the latency of the first P50. The amplitudes were measured from the preceding negativity. The ratio of the second P50 amplitude divided by the first P50 amplitude was calculated for each subject (S2/S1). The amplitudes of the first P50 and the amplitude ratios were compared between the groups (Student’s t test). Even though the age difference between the groups was not significant (t=1.5, df=32, p=0.14), its influence on the amplitude ratios in addition to diagnosis was assessed with an analysis of covariance (ANCOVA). The amplitude ratios were separately correlated with age in each group and with the MMSE score in the group of Alzheimer’s disease patients (Pearson’s correlation coefficient). Only the data at Cz are presented here, since the P50 suppression effect is most pronounced at this site.
Results
The amplitude of the P50 wave following the first click did not differ significantly between the groups (t=0.70, df=32, p=0.49). The amplitude ratio, however, was significantly larger in the Alzheimer’s disease group (Table 1), implying a deficit in P50 suppression. The ANCOVA revealed no significant effect of age on the amplitude ratio (F=0.19, df=1, 31, p=0.66). The amplitude ratio was not correlated with disease severity as expressed by the MMSE score (r=0.03, df=15, p=0.90) or with age (r=–0.14, df=15, p=0.59) within the Alzheimer’s disease group. There was also no correlation of the amplitude ratio with age in the comparison group (r=–0.02, df=15, p=0.94).
Discussion
In the present study, Alzheimer’s disease patients showed diminished sensory gating expressed by a deficit in P50 suppression relative to healthy comparison subjects. This result is not in agreement with the only other published study of sensory gating in Alzheimer’s disease, which did not detect a group difference in P50 suppression between patients and comparison subjects (9). This might be explained by that study’s small number of patients with an identifiable P50 after both clicks (N=6), which yields a power of 0.62 to detect a difference of the magnitude as seen in the present study.
P50 suppression is mediated by the alpha-7 subunit of the nicotinic receptor (3). Alzheimer’s disease is characterized by a disturbed presynaptic integrity of the cholinergic system. Consequently, the observed P50 suppression deficit might result from the presynaptic cholinergic deficit. The crucial role of the cholinergic dysfunction for early sensory processing in Alzheimer’s disease has also been shown in terms of a diminished P1, which has been related to a disturbed thalamic cholinergic system (10).
Furthermore, there is evidence for alpha-7 receptor loss in Alzheimer’s disease (4). Perry et al. (11), however, failed to detect alpha-7 receptor loss. From these controversial findings, it might be speculated that only a subgroup of Alzheimer’s disease patients displays alpha-7 receptor loss. This is supported by the observation that the diminished P50 suppression is not related to the cognitive status of the patients, which would be expected if only the presynaptic cholinergic deficits contributed to disturbed sensory gating. Since sensory gating is attributed to greater distractibility and attentional dysfunction, those patients with alpha-7 receptor loss might be at particular risk for behavioral disturbances. Thus, future studies should focus on the relationship between disturbed sensory gating and the vulnerability for behavioral symptoms. P50 suppression might serve as an electrophysiological predictor for the treatment response of noncognitive disturbances to cholinesterase inhibitors that is observed in Alzheimer’s disease (12).
 |
Received June 15, 2000; revisions received Nov. 29, 2000, and Jan. 31, 2001; accepted Feb. 15, 2001. From the Department of Psychiatry, University of Bonn. Address reprint request to Dr. Jessen, Department of Psychiatry, University of Bonn, Sigmund-Freud-Str. 25, 53105 Bonn, Germany; [email protected] (e-mail).
1. Cullum CM, Harris JG, Waldo MC, Smernoff E, Madison A, Nagamoto HT, Griffith J, Adler LE, Freedman R: Neurophysiological and neuropsychological evidence for attentional dysfunction in schizophrenia. Schizophr Res 1993; 10:131-141Crossref, Medline, Google Scholar
2. Karper LP, Freeman GK, Grillon C, Morgan CA III, Charney DS, Krystal JH: Preliminary evidence of an association between sensorimotor gating and distractibility in psychosis. J Neuropsychiatry Clin Neurosci 1996; 8:60-66Crossref, Medline, Google Scholar
3. Luntz-Leybman V, Bickford PC, Freedman R: Cholinergic gating of response to auditory stimuli in rat hippocampus. Brain Res 1992; 587:130-136Crossref, Medline, Google Scholar
4. Freedman R, Hall M, Adler LE, Leonard S: Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry 1995; 38:22-33Crossref, Medline, Google Scholar
5. Burghaus L, Schutz U, Krempel U, de Vos RA, Jansen Steur EN, Wevers A, Lindstrom J, Schroder H: Quantitative assessment of nicotinic acetylcholine receptor proteins in the cerebral cortex of Alzheimer patients. Brain Res Mol Brain Res 2000; 76:385-388Crossref, Medline, Google Scholar
6. Semlitsch HV, Anderer P, Schuster P, Presslich O: A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology 1986; 23:695-703Crossref, Medline, Google Scholar
7. John ER, Prichep LS, Friedman J, Essig-Peppard T: Neurometric classification of patients with different psychiatric disorders, in Statistic and Topography in Quantitative EEG. Edited by Samson-Dollfus D. Amsterdam, Elsevier, 1988, pp 88-95Google Scholar
8. Jerger K, Biggins C, Fein G: P50 suppression is not affected by attentional manipulations. Biol Psychiatry 1992; 31:365-377Crossref, Medline, Google Scholar
9. Fein G, Biggins C, van Dyke C: The auditory P50 response is normal in Alzheimer’s disease when measured via a paired click paradigm. Electroencephalogr Clin Neurophysiol 1994; 92:536-545Crossref, Medline, Google Scholar
10. Buchwald JS, Erwin RJ, Read S, Van Lancker D, Cummings JL: Midlatency auditory evoked responses: differential abnormality of P1 in Alzheimer’s disease. Electroencephalogr Clin Neurophysiol 1989; 74:378-384Crossref, Medline, Google Scholar
11. Perry E, Martin-Ruiz C, Lee M, Griffiths M, Johnson M, Piggott M, Haroutunian V, Buxbaum JD, Nasland J, Davis K, Gotti C, Clementi F, Tzartos S, Cohen O, Soreq H, Jaros E, Perry R, Ballard C, McKeith I, Court J: Nicotinic receptor subtypes in human brain ageing, Alzheimer and Lewy body diseases. Eur J Pharmacol 2000; 393:215-222Crossref, Medline, Google Scholar
12. Mega MS, Masterman DM, O’Connor SM, Barclay TR, Cummings JL: The spectrum of behavioral responses to cholinesterase inhibitor therapy in Alzheimer disease. Arch Neurol 1999; 56:1388-1393Google Scholar



