Substance Use Disorders in Patients With Posttraumatic Stress Disorder: A Review of the Literature
Abstract
OBJECTIVE: Alcohol use disorders and other substance use disorders are extremely common among patients with posttraumatic stress disorder (PTSD). This article reviews studies pertaining to the epidemiology, clinical phenomenology, and pathophysiology of comorbid PTSD and substance use disorders. METHOD: Studies were identified by means of computerized and manual searches. The review of research on the pathophysiology of PTSD and substance use disorders was focused on studies of the hypothalamic-pituitary-adrenal axis and the noradrenergic system. RESULTS: High rates of comorbidity suggest that PTSD and substance use disorders are functionally related to one another. Most published data support a pathway whereby PTSD precedes substance abuse or dependence. Substances are initially used to modify PTSD symptoms. With the development of dependence, physiologic arousal resulting from substance withdrawal may exacerbate PTSD symptoms, thereby contributing to a relapse of substance use. Preclinical work has led to the proposal that in PTSD, corticotropin-releasing hormone and noradrenergic systems may interact such that the stress response is progressively augmented. Patients may use sedatives, hypnotics, or alcohol in an effort to interrupt this progressive augmentation. CONCLUSIONS: Vigorous control of withdrawal and PTSD-related arousal symptoms should be sought during detoxification of patients with comorbid PTSD and substance use disorders. Inclusion of patients with comorbid PTSD and substance use disorders in neurobiologic research and in clinical trials will be critical for development of effective treatments for this severely symptomatic patient population.
Substance use disorders, particularly abuse of and dependence on central nervous system (CNS) depressants, are common in patients with posttraumatic stress disorder (PTSD). This article reviews clinical, epidemiologic, and neurobiologic studies relevant to the problem of comorbid PTSD and substance use disorders and discusses the clinical implications of these findings.
Clinical Phenomenology and Epidemiology
PTSD develops in some people after exposure to a severe traumatic event. The DSM-IV diagnosis of PTSD consists of symptoms in three clusters: 1) reexperiencing symptoms, including intrusive recollections of the trauma that are triggered by exposure to cues symbolizing the trauma; 2) avoidance symptoms, which involve diminished participation in activities and avoidance of thoughts, people, places, and memories associated with the trauma; and 3) arousal symptoms, which include difficulty sleeping, irritability, difficulty concentrating, hypervigilance, and exaggerated startle response.
Although intoxication and withdrawal symptoms vary across abused substances, all substance use disorders share key features. They include a maladaptive pattern of substance use leading to failure to fulfill work, school, or home obligations; legal problems; and substance-related interpersonal problems. Substance dependence further includes tolerance, withdrawal symptoms upon cessation of use, unsuccessful efforts to control use, and continued use despite persistent substance-related physical or psychological problems.
Persons with PTSD have elevated rates of comorbid psychiatric disorders. Studies of both combat veterans and civilians with PTSD have demonstrated that, among men with PTSD, alcohol abuse or dependence is the most common co-occurring disorder, followed by depression, other anxiety disorders, conduct disorder, and nonalcohol substance abuse or dependence (1, 2). Among women with PTSD, rates of comorbid depression and other anxiety disorders are highest, followed by alcohol abuse and dependence (1, 2). High rates of comorbidity of PTSD and substance use disorders were first reported in war-related studies, in which as many as 75% of combat veterans with lifetime PTSD also met criteria for alcohol abuse or dependence (2). Among civilian populations, estimates of the prevalence of lifetime substance use disorders have ranged from 21.6% to 43.0% in persons with PTSD, compared with 8.1% to 24.7% in persons without PTSD (1, 3, 4). Similarly, among substance abusers in the general population, the reported rate of PTSD is 8.3% (5). Rates of PTSD appear to be higher among patients in inpatient substance abuse treatment (up to 42.5%) (6) and among pregnant women in residential treatment for substance abuse (62%) (7). Surveys of substance-dependent adolescents have also found rates of PTSD ranging up to 19.2% (8).
Patients with both PTSD and a substance use disorder have significantly higher rates of comorbid axis I and II disorders, psychosocial and medical problems, substance- or alcohol-related inpatient admissions, and relapse to substance use, compared with patients whose substance use is not complicated by PTSD (4, 9). Furthermore, patients with PTSD and substance use disorders tend to suffer from more severe PTSD symptoms, particularly those in the avoidance and arousal symptom clusters, than do patients with PTSD alone (10). Conversely, one longitudinal study of patients with PTSD and a comorbid substance use disorder found at 6-month posttreatment follow-up that patients whose PTSD symptoms had remitted reported significantly less substance use than did patients with unremitted PTSD (11).
Relationship of Substance Use to PTSD Symptoms
Elevated rates of comorbid depressive and anxiety disorders in patients with PTSD greatly complicate any effort to develop a model of the relationship between PTSD and substance use. High rates of comorbidity suggest that PTSD and substance use disorders are functionally related to one another. Two primary pathways have been described to explain these high rates of comorbidity. In the first, substance abuse precedes PTSD. To sustain their habit, some substance abusers repetitively place themselves in dangerous situations and, as a result, experience high levels of physical and psychological trauma (5). For example, in a study of patients with PTSD and comorbid cocaine abuse, patients whose cocaine abuse developed first later developed PTSD as a result of trauma sustained in the context of procurement and use of cocaine (12). Given that chronic substance use can lead to higher levels of arousal and anxiety as well as to sensitization of neurobiologic stress systems (13), substance abuse may result in a higher level of vulnerability to development of PTSD after exposure to trauma.
In the second pathway, PTSD precedes development of substance use disorders. In this model, the use of substances is a form of self-medication. Patients report that CNS depressants, such as alcohol, cannabis, opioids, and benzodiazepines acutely improve PTSD symptoms (14). Consistent with this, patients with PTSD report that onset and severity of substance abuse parallel the onset and escalation of PTSD symptoms (14). In addition, clinical evidence suggests that the choice of substances of abuse (CNS depressants versus CNS stimulants) may stem from the particular constellation of PTSD symptoms that patients experience. For example, PTSD patients with alcohol dependence exhibit significantly more arousal symptoms that do PTSD patients with cocaine dependence (10).
In the second model, withdrawal from substances, particularly CNS depressants, may initiate a cycle that perpetuates relapse and continued substance use. The withdrawal syndromes associated with many CNS depressants overlap extensively with the arousal symptoms of PTSD (15) (Figure 1). Substances may be taken initially to ameliorate PTSD symptoms. As noted earlier, patients with PTSD have reported that CNS depressants acutely provide symptom relief (14). Furthermore, objectively measured startle responses are reduced by alcohol (16). However, the physiologic arousal resulting from substance withdrawal may have an additive effect with arousal symptoms stemming from PTSD. The resulting hyperaroused state may serve as a conditioned reminder of traumatic events and thus precipitate an increase in reexperiencing symptoms. Exacerbation of PTSD symptoms may then prompt relapse to substance use in an attempt to self-medicate. Thus, for the PTSD patient who already has symptoms of arousal, the additional arousal that accompanies withdrawal from substances may be intolerable. Alternatively, substances may be used to cope with the traumatic event itself (17). This pattern may particularly apply when trauma that leads to PTSD occurs during adulthood. The initial calming effects from substance use may cue patients to resume substance use when PTSD symptoms reemerge.
Most published data support the second model, in which substance use follows or parallels traumatic exposure and the development of PTSD (18). In a longitudinal study conducted by Chilcoat and Breslau (19), 1,007 adults were reevaluated 3 and 5 years after an initial assessment. The researchers found that preexisting substance abuse did not increase subjects’ risk of subsequent exposure to trauma or their risk of developing PTSD after exposure to trauma. The relationship between exposure to trauma and increased risk for development of a substance use disorder was found to be specific to PTSD, as exposure to trauma without subsequent development of PTSD did not increase risk for development of a substance use disorder (19). Of note, one study of patients with cocaine dependence and PTSD found that patients in whom PTSD preceded the onset of cocaine use were significantly more likely to suffer from comorbid major depression and to use benzodiazepines and opiates than were patients in whom PTSD developed after the onset of cocaine use (12).
Pathophysiology
Our review of the literature on the pathophysiologic basis of comorbid PTSD and addiction selectively focuses on studies of the hypothalamic-pituitary-adrenal (HPA) axis and the noradrenergic system, as these have been most extensively studied in PTSD. It must be emphasized that many other neurobiological systems are involved in both the acute and chronic adaptation to stress and to substance use. These systems include the dopaminergic, γ-aminobutyric acid, benzodiazepine, and serotonergic systems, as well as the thyroid axis. Interactions among these systems in patients with comorbid PTSD and substance dependence are enormously complex. Thus, the potential relationships we discuss between the HPA axis, the noradrenergic system, and symptoms in patients with comorbid PTSD and substance use disorders should be viewed as one part of a far more complex whole.
HPA Axis in PTSD and Addiction
In humans and animals, acute stress elicits a cascade of neurohormonal events, including increased turnover of norepinephrine in terminal projection regions of the locus ceruleus and liberation of hypothalamic corticotropin-releasing hormone (CRH) into the pituitary portal system, which stimulates release of ACTH from the pituitary, which in turn triggers release of cortisol (human) or corticosterone (rat) from the adrenals (20). Animal and human research has implicated this cascade in the pathophysiology of both substance use disorders and PTSD.
Humans with substance dependence most frequently identify stress and negative mood states as reasons for relapse and ongoing substance abuse (21). Recently, a personalized stress imagery task was shown to reliably increase cocaine craving and salivary cortisol in cocaine-dependent patients (22). Animal studies have shown that stress induces relapse to heroin and to cocaine self-administration in rats trained to self-administer these substances and then subjected to a prolonged drug-free period (23, 24). Similarly, in animals naive to illicit substances, a large range of stressors increases the proclivity toward drug self-administration (25). Initial work on the pathophysiology of this phenomenon indicated that stress-induced or stress-enhanced drug self-administration is mediated by corticosterone (26).
Evidence has accumulated to support a role for CRH in mediating the effects of stress on drug self-administration. Central, but not peripheral, administration of CRH has been shown to induce a long-lasting enhancement (sensitization) of the locomotor response to d-amphetamine (27), and pretreatment with a CRH antagonist has been shown to block the development of stress-induced sensitization to d-amphetamine (28). Indeed, central administration of anti-CRH antibody or the CRH receptor antagonist α-helical CRH has been found to block the locomotor hyperactivity induced by cocaine (29).
Withdrawal from chronic cocaine or alcohol administration in rats produces anxiety-like behavior and decreased exploration that is associated with selective increases in CRH in the hypothalamus, amygdala, and basal forebrain (30, 31). Pretreatment with anti-CRH immunoserum or α-helical CRH, blocking the effects of CRH, completely prevents the development of these withdrawal-associated behaviors (30). Consistent with these observations, CSF CRH is elevated in humans in acute alcohol withdrawal and then normalizes or decreases below normal levels with extended abstinence and resolution of withdrawal symptoms (32). Shaham and colleagues (33) found that intracerebroventricular injection of CRH reinstated heroin seeking after extinction in rats trained to self-administer the drug. In addition, α-helical CRH attenuated the reinstatement effect of footshock stress (33). Neither adrenalectomy nor chronic or acute exposure to the corticosterone synthesis inhibitor metyrapone interfered with the reinstatement effects of priming injections of heroin or of footshock stress. A potent, selective CRF1 receptor antagonist, CP-154,526, has been found to attenuate reinstatement of drug seeking induced by footshock stress after up to 14 days of extinction in rats trained to self-administer heroin or cocaine (34).
Findings from both animal and human studies of the effects of chronic stress or of PTSD on HPA axis function vary depending on the experimental paradigm used or the population studied. In patients with PTSD, elevated (35), reduced (36), and normal (37) levels of cortisol secretion have been reported. A series of studies performed by Yehuda and colleagues demonstrated that patients with PTSD have an elevated number of lymphocyte glucocorticoid receptors (38), enhanced suppression of cortisol after administration of dexamethasone (39), a greater than normal decrease in the number of lymphocyte glucocorticoid receptors after administration of dexamethasone (39), and higher than normal increases in ACTH after metyrapone blockade of cortisol synthesis (40). All of these findings suggest that glucocorticoid negative feedback is enhanced in PTSD.
Animal studies examining the effects of uncontrollable stress on HPA axis function have reported initial increases of corticosterone secretion, followed by normalization of corticosterone secretion with ongoing chronic stress (41). However, some investigators have failed to demonstrate normalization of corticosterone secretion with chronic uncontrollable stress (42), particularly in animals that have been reared under stressful conditions (43) or when levels of chronic stress are high (44). In a pattern similar to that found in humans with PTSD, animals subjected to a single episode of prolonged stress and then briefly restressed after a stress-free period showed enhancement of glucocorticoid negative feedback (45).
Although both animal and human studies have suggested that glucocorticoid negative feedback may be enhanced in PTSD, the implications of these observations for CRH secretion in this disorder are unclear. As noted earlier, CRH-producing cells and CRH receptors exist both in the hypothalamus and in extrahypothalamic sites. Findings from some studies have suggested that hypothalamic and extrahypothalamic CRH-producing cells may respond differently to corticosterone. Specifically, corticosterone appears to restrain hypothalamic CRH-producing cells while stimulating extrahypothalamic CRH-producing cells, particularly those in the amygdala (46). Replacement of corticosterone in adrenalectomized rats decreases CRH production in the parvocellular nucleus of the hypothalamus while increasing CRH production in the central nucleus of the amygdala (47). This region-specific pattern of regulation is also seen in adrenally intact rats treated with high-stress levels of corticosterone for extended periods of time (48). Thus, while glucocorticoid feedback may decrease CRH production and release in the hypothalamus, it may stimulate CRH production and release in other brain regions, including the amygdala. This possibility has been addressed in two studies of patients with PTSD, one that examined CSF concentrations of CRH at a single time point (49) and one that examined CSF concentrations of CRH at serial time points over a 6-hour period (37). Both found significantly higher levels of CSF CRH in patients with PTSD than in normal comparison subjects. However, although elevated CSF CRH suggests that brain CRH may be elevated, the specific brain tissues producing CRH elevations cannot be determined from CSF data alone.
The possibility that brain CRH levels are elevated in PTSD is of great interest because of a rich preclinical literature indicating that elevated levels of CRH in the brain, particularly in the amygdala, potentiate fear-related behavioral responses, including the startle response (50). These anxiogenic effects of CRH are reversed by administration of CRH antagonists (50). As noted earlier, findings from animal and human studies have supported a role for CRH in mediating some effects of drugs of abuse, including stress- or priming-induced relapse to drug self-administration and symptoms of withdrawal (27, 28, 32–34). Thus, elevated levels of CRH in the brain in PTSD may mediate both the symptoms of hyperarousal as well as the increased risk for substance abuse and dependence seen in this disorder. More specifically, elevated levels of CRH in the brain in PTSD may enhance the euphorigenic properties of certain drugs, such as stimulants, and may worsen the severity of withdrawal symptoms, thereby prompting patients to relapse to drug use. Conversely, brain CRH elevations induced by withdrawal from substance use may exacerbate symptoms of hyperarousal, which could trigger other symptoms of PTSD, prompting relapse to substance use.
Noradrenergic System in PTSD and Addiction
During chronic uncontrollable stress, norepinephrine turnover increases in specific brain regions, including the locus ceruleus, hypothalamus, hippocampus, amygdala, and cerebral cortex (51). Evidence for noradrenergic dysregulation in patients with PTSD has included elevated 24-hour urinary epinephrine and norepinephrine excretion, a lower than normal number of platelet α2-adrenergic receptors, elevated 24-hour plasma norepinephrine, and exaggerated cardiovascular and 3-methoxy-4-hydroxyphenylglycol (MHPG) (a norepinephrine metabolite) responses to intravenous yohimbine (52). Noradrenergic dysregulation has also been reported during states of withdrawal from chronic self-administration of alcohol and other abused substances. The levels of noradrenaline, norepinephrine, and MHPG in both plasma and CSF have been found to be increased and the number of platelet α2-adrenergic receptors decreased in alcoholics during acute withdrawal (53, 54). The severity of alcoholic withdrawal symptoms has been positively correlated with the concentration of MHPG in CSF (54). Evidence for noradrenergic dysregulation in opiate withdrawal has included findings of elevated plasma MHPG in humans and elevated plasma and brain MHPG in animals (55, 56). In animals, the level of noradrenergic activity was significantly correlated with the severity of withdrawal symptoms (56). These findings have prompted the use of the α2-adrenergic receptor agonist clonidine in the treatment of both opiate withdrawal symptoms and PTSD (57, 58).
Noradrenergic System/HPA Axis Interactions
Evidence that brain CRH and noradrenergic systems modulate each other has been reported. Stress has been shown to increase CRH levels in the locus ceruleus (59), a primary source of noradrenergic projections to all cortices as well as to the thalamus and hypothalamus, while intraventricular administration of CRH has been found to increase the discharge rates of locus ceruleus neurons and to increase norepinephrine turnover in hippocampus, hypothalamus, and prefrontal cortex (60–62). Conversely, stress-induced activation of the locus ceruleus has been blocked by administration of CRH antagonists (63). Similar evidence exists for the interaction of the CRH and noradrenergic systems in the hypothalamus (64) and the amygdala, where stress induces increases in both CRH and norepinephrine (65). Furthermore, norepinephrine in the amygdala appears to stimulate release of CRH (66).
These observations have prompted the proposal by Koob (20) that interactions of the CRH and noradrenergic systems in the brain may, under some conditions, function as a feed-forward system, leading to the progressive augmentation of the stress response with repeated stress exposure that is characteristic of PTSD. This progressive augmentation of response with repeated stress has previously been conceptualized as kindling (67). A feed-forward interaction between the CRH and noradrenergic systems may represent one neurobiologic underpinning of both PTSD and substance use disorders. More specifically, stress, including stress related to self-administration of or withdrawal from substances, may stimulate CRH release in the locus ceruleus, leading to activation of the locus ceruleus and release of norepinephrine in the cortex, which in turn may stimulate the release of CRH in the hypothalamus and amygdala (20). Such an interaction between the brain noradrenergic and CRH systems may mediate the symptoms of hyperarousal seen in PTSD, including exaggerated startle response. The proclivity toward misuse of CNS depressants by patients with PTSD may reflect an attempt to interrupt this feed-forward interaction by suppressing activity of the locus ceruleus with these agents (68).
Conclusions
Clinical and epidemiologic studies confirm that comorbidity of PTSD with substance use disorders is common and that the symptoms of patients with this comorbidity tend to be more severe and more refractory to treatment than those of patients suffering from either disorder alone. Despite the frequency with which patients with both diagnoses present for treatment, no systematic treatment approach of proven efficacy has been developed for this population. Furthermore, little is known about the impact on substance use disorder outcomes of the medications and psychosocial interventions commonly used to treat PTSD, or vice versa.
These limitations notwithstanding, the research conducted to date can inform both clinical practice and future clinical and preclinical research. For example, clinical research suggests that PTSD patients with substance dependence, particularly those who are addicted to CNS depressants, may find the physiologic arousal resulting from substance withdrawal intolerable due to additive effects with preexisting arousal symptoms related to PTSD. Successful detoxification of these patients may thus require inpatient admission to permit vigorous control of withdrawal and PTSD-related arousal symptoms.
Neurobiologic research indicates that high levels of CRH in the brain, particularly in the amygdala, may be common to both PTSD and to substance withdrawal states. Further, CRH antagonists reduce both the anxiety and the enhanced response to illicit substances (sensitization) that are induced by higher levels of brain CRH. These observations suggest that CRH antagonists could potentially have a role in the treatment of patients with PTSD and comorbid substance dependence. Although at present no CRH antagonist has been approved for human use, a series of CRH antagonists that can be administered peripherally have been developed and have been shown to cross the blood brain barrier (34, 69). These agents will be important tools for further defining the potential role of CRH antagonism in the treatment of patients with PTSD and substance dependence and will hopefully lead to development of orally active preparations.
Evidence of noradrenergic dysregulation in both PTSD and in withdrawal from CNS depressants has prompted the use of the α2-adrenoceptor agonist clonidine in both disorders (57, 58). Data from both preclinical and clinical research suggest that this agent, as well as the selective α2-adrenoceptor agonist guanfacine, would be effective in reducing noradrenergic hyperactivity in patients with PTSD and comorbid substance dependence. Guanfacine, given its greater selectivity, may offer a more favorable side effect profile. Given the dearth of established treatments for this patient population, controlled clinical trials to establish the efficacy of these agents are clearly indicated.
Finally, although preclinical work has resulted in considerable progress toward delineating the contributions of the HPA axis and noradrenergic systems to the pathophysiologic underpinnings of PTSD with comorbid substance dependence, few neurobiologic studies have been conducted in this patient population. The inclusion of subjects with this comorbidity may render such studies more complicated, but the data emerging from this work would better inform the clinical management of the difficult-to-treat symptoms of these frequently encountered patients. At the minimum, patients who participate in studies of PTSD or of substance dependence must be thoroughly evaluated for the presence of this comorbidity to permit adequate control of the effects of the comorbid condition on the neurobiologic processes under study.
Received May 11, 2000; revision received Aug. 22, 2000; accepted Nov. 17, 2000. From the Department of Psychiatry, Yale University School of Medicine, New Haven, Conn., and the VA Connecticut Healthcare System. Address correspondence to Dr. Jacobsen, Department of Psychiatry (116A), VA Connecticut Healthcare System, Yale University–West Haven Campus, 950 Campbell Ave., West Haven, CT 06516; [email protected] (e-mail). Supported in part by grants DA-00167, DA-04060, and DA-09250 from the National Institute on Drug Abuse.
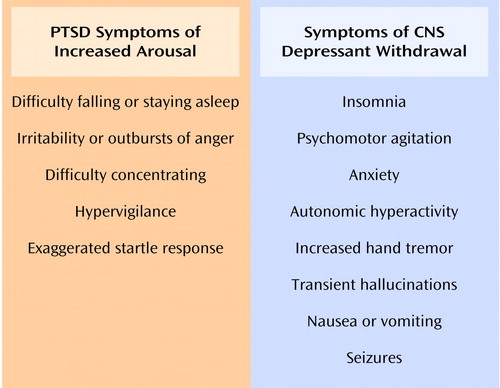
Figure 1. Symptoms of Increased Arousal in PTSD and Symptoms Associated With Withdrawal From CNS Depressantsa
aFrom the DSM-IV criteria for PTSD, alcohol withdrawal, and sedative, hypnotic, or anxiolytic withdrawal.
1. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB: Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1995; 52:1048-1060Google Scholar
2. Kulka RA, Schlenger WE, Fairbank JA, Hough RL, Jordan BK, Marmar CR, Weiss DS: Trauma and the Vietnam War Generation: Report of Findings From the National Vietnam Veterans Readjustment Study. New York, Brunner/Mazel, 1990Google Scholar
3. Breslau N, Davis GC, Andreski P, Peterson E: Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry 1991; 48:216-222Crossref, Medline, Google Scholar
4. Breslau N, Davis GC, Peterson EL, Schultz L: Psychiatric sequelae of posttraumatic stress disorder in women. Arch Gen Psychiatry 1997; 54:81-87Crossref, Medline, Google Scholar
5. Cottler LB, Compton WM III, Mager D, Spitznagel EL, Janca A: Posttraumatic stress disorder among substance users from the general population. Am J Psychiatry 1992; 149:664-670Link, Google Scholar
6. Dansky BS, Saladin ME, Brady KT, Kilpatrick DG, Resnick HS: Prevalence of victimization and posttraumatic stress disorder among women with substance use disorders: comparison of telephone and in-person assessment samples. Int J Addict 1995; 30:1079-1099Google Scholar
7. Thompson MP, Kingree JB: The frequency and impact of violent trauma among pregnant substance abusers. Addict Behav 1998; 23:257-262Crossref, Medline, Google Scholar
8. Deykin EY, Buka SL: Prevalence and risk factors for posttraumatic stress disorder among chemically dependent adolescents. Am J Psychiatry 1997; 154:752-757Link, Google Scholar
9. Najavits LM, Gastfriend DR, Barber JP, Reif S, Muenz LR, Blaine J, Frank A, Crits-Christoph P, Thase M, Weiss RD: Cocaine dependence with and without PTSD among subjects in the National Institute on Drug Abuse Collaborative Cocaine Treatment Study. Am J Psychiatry 1998; 155:214-219Abstract, Google Scholar
10. Saladin ME, Brady KT, Dansky BS, Kilpatrick DG: Understanding comorbidity between PTSD and substance use disorders: two preliminary investigations. Addict Behav 1995; 20:643-655Crossref, Medline, Google Scholar
11. Ouimette PC, Brown PJ, Najavits LM: Course and treatment of patients with both substance use and posttraumatic stress disorders. Addict Behav 1998; 23:785-795Crossref, Medline, Google Scholar
12. Brady KT, Dansky BS, Sonne SC, Saladin ME: Posttraumatic stress disorder and cocaine dependence. Am J Addict 1998; 7:128-135Crossref, Medline, Google Scholar
13. Aouizerate B, Schluger JH, Perret G, McClary K, Ho A, Piazza PV, Kreek MJ: Enhanced sensitivity to negative glucocorticoid feedback in methadone patients with ongoing cocaine dependence, in Proceedings of the College on Problems of Drug Dependence Annual Meeting. Bethesda, Md, National Institute on Drug Abuse, CPDD, 1998, p 3Google Scholar
14. Bremner JD, Southwick SM, Darnell A, Charney DS: Chronic PTSD in Vietnam combat veterans: course of illness and substance abuse. Am J Psychiatry 1996; 153:369-375Link, Google Scholar
15. van der Kolk B, Greenberg M, Boyd H, Krystal J: Inescapable shock, neurotransmitters, and addiction to trauma: toward a psychobiology of post traumatic stress. Biol Psychiatry 1985; 20:314-325Crossref, Medline, Google Scholar
16. Hutchison KE, Rohsenow D, Monti P, Palfai T, Swift R: Prepulse inhibition of the startle reflex: preliminary study of the effects of a low dose of alcohol in humans. Alcohol Clin Exp Res 1997; 21:1312-1319Google Scholar
17. Mirin SM, McKenna GJ: Combat zone adjustment: the role of marihuana use. Mil Med 1975; 140:482-485Crossref, Medline, Google Scholar
18. Keane TM, Gerardi RJ, Lyons JA, Wolfe J: The interrelationship of substance abuse and posttraumatic stress disorder: epidemiological and clinical considerations. Recent Dev Alcohol 1988; 6:27-48Medline, Google Scholar
19. Chilcoat HD, Breslau N: Posttraumatic stress disorder and drug disorders: testing causal pathways. Arch Gen Psychiatry 1998; 55:913-917Crossref, Medline, Google Scholar
20. Koob GF: Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry 1999; 46:1167-1180Google Scholar
21. Brewer DD, Catalano RF, Haggerty K, Gainey RR, Fleming CB: A meta-analysis of predictors of continued drug use during and after treatment for opiate addiction. Addiction 1998; 93:73-92Crossref, Medline, Google Scholar
22. Sinha R, Catapano D, O’Malley S: Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) 1999; 142:343-351Crossref, Medline, Google Scholar
23. Shaham Y, Stewart J: Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology (Berl) 1995; 119:334-341Crossref, Medline, Google Scholar
24. Erb S, Shaham Y, Stewart J: Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology (Berl) 1996; 128:408-412Crossref, Medline, Google Scholar
25. Piazza PV, Deminiere JM, Le Moal M, Simon H: Stress- and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Res 1990; 514:22-26Crossref, Medline, Google Scholar
26. Deroche V, Marinelli M, LeMoal M, Piazza PV: Glucocorticoids and behavioral effects of psychostimulants, II: cocaine intravenous self-administration and reinstatement depend on glucocorticoid levels. J Pharmacol Exp Ther 1997; 281:1401-1407Google Scholar
27. Cador M, Cole BJ, Koob GF, Stinus L, Le Moal M: Central administration of corticotropin releasing factor induces long-term sensitization to d-amphetamine. Brain Res 1993; 606:181-186Crossref, Medline, Google Scholar
28. Cole BJ, Cador M, Stinus L, Rivier J, Vale W, Koob GF, Le Moal M: Central administration of a CRF antagonist blocks the development of stress-induced behavioral sensitization. Brain Res 1990; 512:343-346Crossref, Medline, Google Scholar
29. Sarnyai Z, Hohn J, Szabo G, Penke B: Critical role of endogenous corticotropin-releasing factor (CRF) in the mediation of the behavioral action of cocaine in rats. Life Sci 1992; 51:2019-2024Google Scholar
30. Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G: Brain corticotropin-releasing factor mediates “anxiety-like” behavior induced by cocaine withdrawal in rats. Brain Res 1995; 675:89-97Crossref, Medline, Google Scholar
31. Merlo-Pich E, Koob GF, Heilig M, Menzaghi F, Vale W, Weiss F: Corticotropin-releasing factor release from mediobasal hypothalamus of the rat as measured by microdialysis. Neuroscience 1993; 55:695-707Crossref, Medline, Google Scholar
32. Adinoff B, Anton R, Linnoila M, Guidotti A, Nemeroff CB, Bissette G: Cerebrospinal fluid concentrations of corticotropin-releasing hormone (CRH) and diazepam-binding inhibitor (DBI) during alcohol withdrawal and abstinence. Neuropsychopharmacology 1996; 15:288-295Crossref, Medline, Google Scholar
33. Shaham Y, Funk D, Erb S, Brown TJ, Walker C-D, Stewart J: Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci 1997; 17:2605-2614Google Scholar
34. Shaham Y, Erb S, Leung S, Buczek Y, Stewart J: CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor 1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berl) 1998; 137:184-190Crossref, Medline, Google Scholar
35. Maes M, Lin A, Bonaccorso S, van Hunsel F, Van Gastel A, Delmeire L, Biondi M, Bosmans E, Kenis G, Scharpe S: Increased 24-hour urinary cortisol excretion in patients with post-traumatic stress disorder and patients with major depression, but not in patients with fibromyalgia. Acta Psychiatr Scand 1998; 98:328-335Crossref, Medline, Google Scholar
36. Mason JW, Giller EL, Kosten TR, Ostroff RB, Podd L: Urinary free-cortisol levels in posttraumatic stress disorder patients. J Nerv Ment Dis 1986; 174:145-149Crossref, Medline, Google Scholar
37. Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, Bruce AB, Orth DN, Geracioti TD Jr: Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry 1999; 156:585-588Abstract, Google Scholar
38. Yehuda R, Lowy MT, Southwick SM, Shaffer D, Giller EL Jr: Lymphocyte glucocorticoid receptor number in posttraumatic stress disorder. Am J Psychiatry 1991; 148:499-504Link, Google Scholar
39. Yehuda R, Boisoneau D, Lowy MT, Giller EL: Dose-response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. Arch Gen Psychiatry 1995; 52:583-593Crossref, Medline, Google Scholar
40. Yehuda R, Levengood RA, Schmeilder J, Wilson S, Guo LS, Gerber D: Increased pituitary activation following metyrapone administration in post-traumatic stress disorder. Psychoneuroendocrinology 1996; 21:1-16Crossref, Medline, Google Scholar
41. Kant GJ, Bauman RA, Anderson SM, Mougey EH: Effects of controllable vs uncontrollable chronic stress on stress-responsive plasma hormones. Physiol Behav 1992; 51:1285-1288Google Scholar
42. Irwin J, Ahluwalia P, Zacharko RM, Anisman H: Central norepinephrine and plasma corticosterone following acute and chronic stressors: influence of social isolation and handling. Pharmacol Biochem Behav 1986; 24:1151-1154Google Scholar
43. Gamallo A, Villanua A, Trancho G, Fraile A: Stress adaptation and adrenal activity in isolated and crowded rats. Physiol Behav 1986; 36:217-221Crossref, Medline, Google Scholar
44. Young EA, Akana S, Dallman MF: Decreased sensitivity to glucocorticoid fast feedback in chronically stressed rats. Neuroendocrinology 1990; 51:536-542Crossref, Medline, Google Scholar
45. Liberzon I, Krstov M, Young EA: Stress-restress: effects on ACTH and fast feedback. Psychoneuroendocrinology 1997; 22:443-453Crossref, Medline, Google Scholar
46. Schulkin J, Gold PW, McEwen BS: Induction of corticotropin-releasing hormone gene expression by glucocorticoids; implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology 1998; 23:219-243Crossref, Medline, Google Scholar
47. Makino S, Gold PW, Schulkin J: Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res 1994; 640:105-112Crossref, Medline, Google Scholar
48. Makino S, Gold PW, Schulkin J: Effects of corticosterone on CRH mRNA and content in the bed nucleus of the stria terminalis; comparison with the effects in the central nucleus of the amygdala and the paraventricular nucleus of the hypothalamus. Brain Res 1994; 657:141-149Crossref, Medline, Google Scholar
49. Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS: Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry 1997; 154:624-629Link, Google Scholar
50. Swerdlow NR, Britton KT, Koob GF: Potentiation of acoustic startle by corticotropin-releasing factor (CRF) and by fear are both reversed by alpha-helical CRF (9-41). Neuropsychopharmacology 1989; 2:285-292Crossref, Medline, Google Scholar
51. Tanaka T, Yokoo H, Mizoguchi K, Yoshida M, Tsuda A, Tanaka M: Noradrenaline release in the rat amygdala is increased by stress: studies with intracerebral microdialysis. Brain Res 1991; 544:174-176Crossref, Medline, Google Scholar
52. Southwick SM, Bremner JD, Rasmusson A, Morgan CA, Arnsten A, Charney DS: Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry 1999; 46:1192-1204Google Scholar
53. Smith AJ, Brent PJ, Henry DA, Foy A: Plasma noradrenaline, platelet alpha 2-adrenoceptors, and functional scores during ethanol withdrawal. Alcohol Clin Exp Res 1990; 14:497-502Crossref, Medline, Google Scholar
54. Hawley RJ, Major LF, Schulman EA, Linnoila M: Cerebrospinal fluid 3-methoxy-4-hydroxyphenylglycol and norepinephrine levels in alcohol withdrawal: correlations with clinical signs. Arch Gen Psychiatry 1985; 42:1056-1062Google Scholar
55. Charney DS, Redmond DE, Galloway MP, Kleber HD, Heninger GR, Murberg M, Roth RH: Naltrexone precipitated opiate withdrawal in methadone addicted human subjects: evidence for noradrenergic hyperactivity. Life Sci 1984; 35:1263-1272Google Scholar
56. Swann AC, Elsworth JD, Charney DS, Jablons DM, Roth RH, Redmond DE, Maas JW: Brain catecholamine metabolites and behavior in morphine withdrawal. Eur J Pharmacol 1982; 86:167-175Crossref, Medline, Google Scholar
57. Agren H: Clonidine treatment of the opiate withdrawal syndrome: a review of clinical trials of a theory. Acta Psychiatr Scand Suppl 1986; 327:91-113Medline, Google Scholar
58. Harmon RJ, Riggs PD: Clonidine for posttraumatic stress disorder in preschool children. J Am Acad Child Adolesc Psychiatry 1996; 35:1247-1249Google Scholar
59. Chappell PB, Smith MA, Hilts CD, Bissette G, Ritchie J, Andersen C, Nemeroff CB: Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J Neurosci 1986; 10:2908-2916Google Scholar
60. Valentino RJ, Foote SL, Aston-Jones G: Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Res 1983; 270:363-367Crossref, Medline, Google Scholar
61. Zhang JJ, Swiergiel AH, Palamarchouk VS, Dunn AJ: Intracerebroventricular infusion of CRF increases extracellular concentrations of norepinephrine in the hippocampus and cortex as determined by in vivo voltametry. Brain Res Bull 1998; 47:277-284Crossref, Medline, Google Scholar
62. Lavicky J, Dunn AJ: Corticotropin-releasing factor stimulates catecholamine release in hypothalamus and prefrontal cortex in freely moving rats as assessed by microdialysis. J Neurochem 1993; 60:602-612Crossref, Medline, Google Scholar
63. Valentino RJ, Page ME, Curtis AL: Activation of noradrenergic locus coeruleus neurons by hemodynamic stress is due to local release of corticotropin-releasing factor. Brain Res 1991; 555:25-34Crossref, Medline, Google Scholar
64. Pacak K, Palkovits M, Kopin IJ, Goldstein DS: Stress-induced norepinephrine release in the hypothalamic paraventricular nucleus and pituitary-adrenocortical and sympathoadrenal activity: in vivo microdialysis studies. Front Neuroendocrinol 1995; 16:89-150Crossref, Medline, Google Scholar
65. Pich EM, Lorang M, Yeganeh M, Rodriquez de Fonseca F, Raber J, Koob GF, Weiss F: Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci 1995; 15:5439-5447Google Scholar
66. Raber J, Koob GF, Bloom FE: Interleukin-2 (IL-2) induces corticotropin-releasing factor (CRF) release from the amygdala and involves a nitric oxide-mediated signaling: comparison with the hypothalamic response. J Pharmacol Exp Ther 1995; 272:815-824Medline, Google Scholar
67. Post RM, Weiss SRB, Smith M, Li H, McCann U: Kindling versus quenching; implications for the evolution and treatment of posttraumatic stress disorder. Ann NY Acad Sci 1997; 821:285-295Crossref, Medline, Google Scholar
68. Kosten TR, Krystal J: Biological mechanisms in posttraumatic stress disorder: relevance for substance abuse. Recent Dev Alcohol 1988; 6:49-68Medline, Google Scholar
69. Arai K, Ohata H, Shibasaki T: Non-peptidic corticotropin-releasing hormone receptor type 1 antagonist reverses restraint stress-induced shortening of sodium pentobarbital-induced sleeping time of rats: evidence that an increase in arousal induced by stress is mediated through CRH receptor type 1. Neurosci Lett 1998; 255:103-106Crossref, Medline, Google Scholar



