Altered Serotonin 2A Receptor Activity in Women Who Have Recovered From Bulimia Nervosa
Abstract
OBJECTIVE: The authors’ goal was to confirm that brain serotonin (5-HT) alterations are present in patients who have recovered from bulimia nervosa. Positron emission tomography imaging with [18F]altanserin was used to characterize binding of the 5-HT2A receptor, which might contribute to altered feeding, mood, or impulse control. METHOD: Nine women who had recovered from bulimia nervosa (they had no episodes of binge eating or purging, were at normal weight, and had regular menstrual cycles for more than 1 year) were compared with 12 female volunteers who had never had bulimia. RESULTS: The healthy volunteers, but not the women who had recovered from bulimia nervosa, had an age-related decline in 5-HT2A binding. Women who had recovered from bulimia nervosa had a reduction of medial orbital frontal cortex 5-HT2A binding. CONCLUSIONS: The lack of age-related changes in 5-HT activity is further evidence of 5-HT alterations in subjects who have recovered from bulimia nervosa. In addition, vulnerabilities for eating disorders, impulse dyscontrol, and mood disturbances may involve 5-HT and frontal lobe activity.
Bulimia nervosa usually has an onset in adolescent women who are of normal body weight. It is characterized by restrictive eating alternating with binge eating and purging and body image distortions. Mood disturbances and extremes of impulse control such as impulsive and obsessive behaviors are common (1). Physiological and pharmacological studies support the possibility that altered central nervous system serotonin (5-HT) neurotransmitter activity could contribute to a susceptibility to develop appetitive and behavioral alterations in bulimia nervosa (1). Altered 5-HT activity in bulimia nervosa could be a consequence of pathological dietary behaviors. However, people who have recovered from bulimia nervosa also have 5-HT alterations as well as behavioral symptoms consistent with a dysregulation of 5-HT neuronal pathways (2, 3), raising the possibility that such alterations are trait-related and contribute to the pathogenesis of this disorder.
To further understand 5-HT activity in women who have recovered from bulimia nervosa, we used the radioligand [18F]altanserin, a specific 5-HT2A receptor antagonist, and positron emission tomography (PET) imaging. The 5-HT2A receptor system has been implicated in the modulation of feeding, mood, and anxiety as well as in antidepressant efficacy (4).
Method
Nine women who had previously met DSM-IV criteria for bulimia nervosa were recruited for the study. None of these women had a history of anorexia nervosa, and all had maintained greater than 85% of average body weight since development of an eating disorder. To be considered recovered, for at least 1 year before the study subjects had to 1) maintain a weight above 90% average body weight, 2) have regular menstrual cycles, 3) have had no episodes of binge eating or purging and not engaged in restrictive eating patterns. Additionally, subjects must not have used psychoactive medication such as antidepressants and not met criteria for alcohol or drug abuse or dependence within 3 months of the study.
Twelve comparison women were recruited through local advertisements. Comparison women had no history of an eating disorder or any other psychiatric, medical, or neurological illness; they also had no first-degree relatives with an eating disorder. They had normal menstrual cycles and a normal weight range since menarche. All subjects gave written informed consent. Methods for recruitment of subjects, assessment of lifetime axis I DSM-III-R diagnoses, and current psychopathology have been described elsewhere (2).
PET imaging was done during the first 10 days of the follicular phase for all subjects. Methods for imaging procedures and data analyses have been described elsewhere (5, 6). In brief, magnetic resonance (spoiled gradient recall acquisition) images were acquired, aligned to the PET images, and used for delineation of regions of interest. PET imaging was done with the 5-HT2A receptor-specific radioligand [18F]altanserin on a Siemens HR+ scanner (Siemens CTI, Knoxville, Tenn.). Repeated arterial blood samples were collected for quantification of plasma radioactivity from [18F]altanserin and metabolite analysis. The Logan graphical analysis (7) was applied to regional specific 5-HT2A binding over 10–90 minutes after tracer injection.
Between-group comparisons were made by using two-tailed group t tests. Levene’s test was used to test homogeneity of variance, and pooled or separate variance t tests were reported as appropriate. Correlations were examined with linear regression analysis. Factors that were considered possible confounds of 5-HT2A receptor binding were explored with a series of two-factor analyses of covariance (ANCOVAs) with one grouping factor (women with recovered bulimia nervosa versus women who never had bulimia). Distribution volume ratio values are expressed as means and standard deviations.
Results
Women who had recovered from bulimia nervosa and comparison women were of similar ages (mean=30 years, SD=4, and mean=27, SD=6, respectively) (t=1.07, df=19, n.s.), current average body weight (mean=110%, SD=14%, and mean=103%, SD=6%) (t=1.43, df=19, n.s.), and low average body weight in the past (mean=95%, SD=12, and mean=97%, SD=7) (t=0.62, df=19, n.s.). Women who had recovered from bulimia nervosa had the onset of bulimia nervosa at a mean age of 16 (SD=1, range=14–18) and had been recovered from bulimia nervosa for a mean of 54 months (SD=44, range=12–156) at the time of this study.
In terms of lifetime axis I diagnoses in recovered bulimia nervosa subjects, five of the nine met criteria for major depression, four for alcohol dependence, and three for obsessive-compulsive disorder (OCD). At the time of the study no subject had a current major depressive episode or alcohol dependence.
Compared with the never-bulimic women, the women who had recovered from bulimia nervosa had significantly higher scores on the Eating Disorders Inventory (mean=52, SD=38, and mean=7, SD=5, respectively) (t=4.18, df=7.1, p<0.001), trait anxiety on the Spielberger State-Trait Anxiety Inventory (mean=41, SD=16, and mean=28, SD=5) (t=2.62, df=8.0, p<0.05), and depression on the Beck Depression Inventory (mean=8, SD=9, and mean=1, SD=2) (t=2.18, df=7.4, p<0.05).
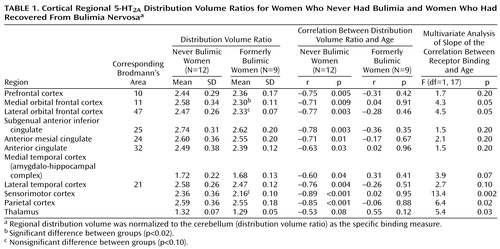
The women who never had bulimia had significant negative relationships between [18F]altanserin binding and age for most cortical regions (Table 1). In contrast, there were no significant relationships between [18F]altanserin binding and age in the women who had recovered from bulimia nervosa. The two groups showed a significant difference in slopes for the thalamus, parietal cortex, and sensorimotor cortex and a nonsignificant difference for the medial and lateral orbital frontal cortex and the medial temporal cortex.
Women who had recovered from bulimia nervosa had a significantly lower level of [18F]altanserin binding for the medial orbital frontal cortex (t=2.7, df=19, p=0.02) than the never bulimic women and a nonsignificantly lower level for the lateral orbital frontal cortex (t=1.7, df=13.8, p=0.09) and sensorimotor cortex (t=1.8, df=13.4, p=0.09). Nonparametric Mann Whitney testing showed a nonsignificant difference for these comparisons. The fraction of unmetabolized [18F]altanserin in plasma was similar in the two groups over the scanning interval. There was no relationship between binding of any region of interest and lifetime axis I diagnoses or other behavioral measures, weight, duration of illness, or recovery from bulimia nervosa.
Discussion
These data confirm and extend the possibility that a disturbance of 5-HT neuronal activity is present after recovery from bulimia nervosa. The women who never had bulimia had an age-related decline in 5-HT2A binding, but 5-HT2A binding was not associated with age in the women who had recovered from bulimia nervosa. Similarly, in an earlier study (2) our group reported a significant inverse relationship between CSF 5-HIAA concentrations and age for nonbulimic women but not for women who had recovered from bulimia nervosa. The minimal overlap in subjects (N=3) between our two studies provides relatively independent replication of group differences in age/serotonin relationships.
Postmortem human studies and PET imaging with 5-HT ligands show a strong inverse correlation between binding of cortical 5-HT2A receptors and age (5, 8, 9). Bulimia nervosa is a largely gender-specific disorder that invariably begins within a narrow postpubertal age range. Whether 5-HT activity in bulimia nervosa is dissociated from normal age-associated changes is speculative, but the speculation raises the question of whether the 5-HT system becomes free-running and insensitive to normal developmental mechanisms.
To our knowledge, this is the first study to question whether a disturbance of 5-HT2A binding occurs in the orbital frontal cortex in women who have recovered from bulimia nervosa. Many people with bulimia nervosa have unstable, dysphoric mood states and disturbances of self-control, such as impulsive and obsessive behaviors (1). Disturbances ofimpulsive and compulsive behaviors are associated with prefrontal cortical function in other psychiatric disorders. For example, PET studies using 2-[18F]fluoro-2-deoxy-D-glucose have found that impulsive aggression disorders have reduced activity of orbital/prefrontal and related areas (10), whereas increased activity has been found in OCD (11). Similarly, serotonergic function has been implicated in behavioral undercontrol and overcontrol, mood, and appetite regulation (12, 13). These disorders may share a common neuronal pathway involving 5-HT and the frontal cortex but have different pathophysiological loci.
Women who have recovered from bulimia nervosa have been found to have elevated CSF 5-HIAA concentrations (2), but it is not known whether this is a consequence of increased extracellular 5-HT. In vitro and animal studies (14) suggest that regionally selective 5-HT2A receptors may be down-regulated in response to increased serotonergic transmission. Thus, altered regional binding of 5-HT2A receptors in recovered bulimia nervosa offers additional support for the hypothesis of increased serotonergic transmission after recovery.
In terms of limitations, the Logan graphical method (7) and similar plasma metabolite concentrations in the two groups argue against group differences in radiometabolites of [18F]altanserin. A relatively large number of statistical analyses were conducted with small numbers of subjects, potentially leading to type I errors. A priori, we thought that regional 5-HT2A binding differences were likely to be in the frontal cortex or related limbic regions, given the evidence implicating these regions in overcontrol and undercontrol and mood disorders. Multiple brain regions were included to demonstrate consistent group differences between 5-HT2A binding and age. Moreover, the effects of laterality or age or psychiatric comorbidity cannot be adequately investigated in a small study group. These data should be considered preliminary. Replication in larger groups of subjects is needed for confirmation.
A cross-sectional design limited the ability to conclude that reduced [18F]altanserin binding is a premorbid trait contributing to the development of these disorders. Moreover, it is possible that the persistence of mild abnormal eating behaviors could have contributed to the findings.
In summary, these data add further support to the possibility that biological vulnerabilities contribute to the pathogenesis of bulimia nervosa.
 |
Received March 16, 2000; revisions received Nov. 7, 2000, and Feb. 22, 2001; accepted March 6, 2001. From the Department of Psychiatry, University of Pittsburgh School of Medicine, Western Psychiatric Institute and Clinic; the Department of Radiology, University of Pittsburgh School of Medicine; Presbyterian University Hospital, Pittsburgh; and the Department of Psychology, Michigan State University School of Medicine, East Lansing. Address reprint requests to Dr. Kaye, University of Pittsburgh, Western Psychiatric Institute and Clinic, 3811 O’Hara St., Pittsburgh, PA 15213; [email protected] (e-mail). Supported in part by NIMH grants MH-42984 (The Neurobiology of Feeding Behavior in Bulimia) and RR-00084 (Children’s Hospital Clinical Research Center, Pittsburgh).
1. Kaye W, Strober M: Neurobiology of eating disorders, in Neurobiology of Mental Illness. Edited by Charney DS, Nestler EJ, Bunney BS. New York, Oxford University Press, 1999, pp 891-906Google Scholar
2. Kaye WH, Greeno CG, Moss H, Fernstrom J, Lilenfeld LR, Weltzin TE, Mann JJ: Alterations in serotonin activity and psychiatric symptoms after recovery from bulimia nervosa. Arch Gen Psychiatry 1998; 55:927-935Crossref, Medline, Google Scholar
3. Smith KA, Fairburn CG, Cowen PJ: Symptomatic relapse in bulimia nervosa following acute tryptophan depletion. Arch Gen Psychiatry 1999; 56:171-176Crossref, Medline, Google Scholar
4. Barnes NM, Sharp T: A review of central 5-HT receptors and their function. Neuropharmacology 1999; 38:1083-1152Google Scholar
5. Meltzer CC, Smith G, Price JC, Reynolds CF, Mathis CA, Greer P, Lopresti B, Mintun MA, Pollock BG, Ben-Eliezer D, Cantwell MN, Kaye W, DeKosky ST: Reduced binding of [18F]altanserin to serotonin type 2A receptors in aging: persistence of effect after partial volume correction. Brain Res 1998; 813:167-171Crossref, Medline, Google Scholar
6. Smith GS, Price JC, Lopresti BJ, Huang Y, Simpson N, Holt D, Mason NS, Meltzer CC, Sweet RA, Nichols T, Sashin D, Mathis CA: Test-retest variability of serotonin 5-HT2A receptor binding measured with positron emission tomography and [18F]altanserin in the human brain. Synapse 1998; 30:380-392Crossref, Medline, Google Scholar
7. Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, MacGregor RR, Hitzemann R, Bendriem B, Gatley, SJ: Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N11C-methyl]-(-)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab 1990; 10:740-747Crossref, Medline, Google Scholar
8. Arango V, Underwood MD, Mann JJ: Postmortem findings in suicide victims: implications for in vivo imaging studies. Ann NY Acad Sci 1997; 836:269-287Crossref, Medline, Google Scholar
9. Lidow MS, Rakic P: Scheduling of monoaminergic neurotransmitter receptor expression in the primate neocortex during postnatal development. Cereb Cortex 1992; 2:401-416Crossref, Medline, Google Scholar
10. Raine A, Phil D, Stoddard J, Bihrle S, Buchsbaum M: Prefrontal glucose deficits in murderers lacking psychosocial deprivation. Neuropsychiatry Neuropsychol Behav Neurol 1998; 11:1-7Medline, Google Scholar
11. Insel TR: Toward a neuroanatomy of obsessive-compulsive disorder. Arch Gen Psychiatry 1992; 49:739-744Crossref, Medline, Google Scholar
12. Stein DJ, Trestman RL, Mitropoulou V, Coccaro EF, Hollander E, Siever LJ: Impulsivity and serotonergic function in compulsive personality disorder. J Neuropsychiatry Clin Neurosci 1996; 8:393-398Crossref, Medline, Google Scholar
13. Mann JJ: Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharmacology 1999; 21:99S-105SCrossref, Medline, Google Scholar
14. Saucier C, Morris SJ, Albert PR: Endogenous serotonin-2A and -2C receptors in Balb/c-3T3 cells revealed in serotonin-free medium: desensitization and down-regulation by serotonin. Biochem Pharmacol 1998; 56:1347-1357Google Scholar



