Sweet Taste Preference as a Risk Factor for Alcohol Dependence
Abstract
OBJECTIVE: Previous research has found that alcoholics have a greater preference for sweet solutions than comparison subjects. This study tested the hypothesis that preference for sweet solutions is a marker for alcoholism risk. METHOD: A total of 122 nonalcoholic subjects (59 men) participated. Fifty-eight subjects had a paternal history of alcoholism, and 64 did not. Each subject rated a series of sucrose solutions for intensity of sweetness and degree of preference. RESULTS: Subjects were able to rate accurately the relative intensity of sweetness in the sucrose solutions. Both subjects with and those without a paternal history of alcoholism preferred a 0.42-M sucrose solution, irrespective of gender. CONCLUSIONS: This study failed to support the hypothesis that sweet preference is a marker of alcoholism risk. The sweet preference observed previously among alcoholics may be a consequence of chronic alcohol consumption or other factors associated with heavy drinking.
Alcohol dependence has a substantial genetic component, as evidenced by family, twin, and adoption studies (1). Efforts to identify markers of alcoholism risk have included studies of taste sensitivity (2), with recent research focusing on the hedonic value of sucrose solutions as a marker of risk. Studies in rodents have shown an association between intake of sweet and ethanol solutions (3–6). Some animal studies (7, 8) (compare with reference 9) have shown that the reinforcing effects of both saccharin and ethanol may be genetically related. However, a study of 311 human twin pairs provided no evidence for a heritable effect on sucrose preference (10).
Kampov-Polevoy et al. (11) reported an association in humans between preference for sweet solutions and alcoholism. They found that a significantly greater proportion of alcoholics presented with a series of sucrose solutions showed a preference for the sweetest (0.83-M) solution. These investigators also reported that family history of alcoholism was associated with a preference for a 0.83-M sucrose solution in a mixed group of alcoholic and nonalcoholic comparison subjects; they interpreted this finding as support for a genetic link between alcoholism and sweet preference (8).
The study reported here examined whether nonalcoholics differentiated by paternal history of alcoholism differed in their preference for sweet-tasting solutions. Since children of an alcoholic father are at a greater risk of developing alcoholism (1), this study tested the hypothesis that sweet preference is a phenotypic marker for alcoholism risk.
Method
A total of 122 healthy subjects were enrolled in the study. Advertising was used to recruit a study group that was nearly equally divided by both family history of alcoholism and sex. Subjects were paid for their participation. The Structured Clinical Interview for DSM-III-R (12) was used to evaluate personal psychiatric history. A family history interview consisting of DSM-III-R criteria was administered to the subject and a family member to ascertain parental history of alcoholism. Exclusion criteria included a lifetime history of a psychiatric or substance use disorder, regular psychoactive medication use, a neurological or endocrine disorder, or significant head trauma. Paternal alcoholism history served to reflect risk; subjects with a maternal history of alcoholism were excluded to avoid a possible confounding effect of prenatal alcohol exposure.
Fifty-eight subjects (47.5%) had a paternal history of alcoholism. Among subjects without a paternal history of alcoholism (N=64, 52.5%), neither parent had a lifetime diagnosis of an alcohol use disorder. In the group with a paternal history of alcoholism, the mean percentage of first-degree and second-degree family members with alcoholism was 35.3% (SD=15.2) and 16.8% (SD=16.0), respectively (compared with mean=0.6%, SD=3.2, and mean=6.9%, SD=11.9, respectively, for subjects without a paternal history of alcoholism). The family history groups were comparable (all p>0.10) with respect to sex (53.4% of those with a paternal history of alcoholism [N=31] and 50.0% of those without a paternal history [N=32] were female), race/ethnicity (82.8% of those with a paternal history [N=48] and 89.1% of those without [N=57] were white), age (mean=26.0 years, SD=5.8, for those with a paternal history and mean=25.8 years, SD=6.1, for those without), and educational level (mean=15.4 years, SD=2.1, for those with a paternal history and mean=16.0 years, SD=2.5, for those without).
After a complete description of the study, subjects were asked to give written informed consent.Subjects’ hedonic responses to the following sucrose concentrations were assessed with methods employed in previous studies (11, 13): 0.05, 0.10, 0.21, 0.42, and 0.83 M. Twenty-five tests were done. The tests were arranged in five blocks containing each of the five solutions, with each block in random order. All subjects received the solutions in the same order.
Immediately after tasting each solution, subjects were instructed to mark their sensitivity and preference ratings on a 200-mm visual analog scale that was anchored with the appropriate descriptors. Sweet liking was defined as preferring a sucrose concentration ≥0.42 M, and sweet disliking was a preference for a concentration ≤0.10 M.
Multivariate analysis of variance was used to determine the main effects of paternal history of alcoholism and sex and the interaction of these factors on the preference ratings for each of the five sucrose solutions. A nonparametric analysis was also performed to permit comparison with the results of a previous study (11).
Results
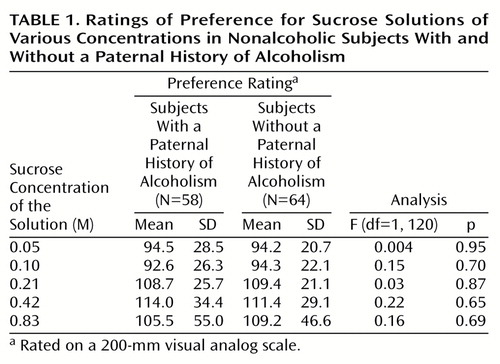
Both subjects with and subjects without a paternal history of alcoholism discriminated among the different sucrose concentrations (r=0.88, N=58, p<0.001, and r=0.87, N=64, p<0.001, respectively). A clear preference was noted for the three sweetest solutions, with the 0.42-M solution given the highest ratings (overall, F=10.0, df=4, 115, p<0.001) (Table 1). The preference ratings for the 0.21-M, 0.42-M, and 0.83-M solutions were statistically indistinct (Bonferroni-adjusted analysis of variance [ANOVA], p>0.05), but all three were significantly more likely to be preferred over the 0.05-M and 0.10-M solutions (Bonferroni-adjusted ANOVA, p<0.05). These results did not differ as a function of paternal history of alcoholism (F=0.71, df=4, 115, p=0.59), sex (F=1.27, df=4, 115, p=0.29), or the interaction of paternal history of alcoholism and sex (F=1.56, df=4, 115, p=0.19).
Of the 122 subjects in the study, 91 (74.6%) were categorized as sweet likers or dislikers. Twenty-five subjects (20.5%) preferred the 0.21-M solution, and six subjects (4.9%) expressed no preference. Among both subjects with and subjects without a paternal history of alcoholism, sweet-liking subjects predominated (70 of 91, 76.9%), although the difference in proportions was not significant (80.0%, N=32 of 40, and 74.5%, N=38 of 51, respectively) (χ2=0.13, df=1, p=0.71, with Yates’s correction).
Discussion
The study results failed to support the hypothesis that sweet preference is a risk factor for the development of alcohol dependence. Both family history groups showed a preference for the 0.42-M solution. This suggests that the effect observed by Kampov-Polevoy et al. (11, 13) in alcoholics may be due to chronic heavy drinking or an associated feature, such as poor nutrition, rather than reflecting an underlying genetic predisposition to the disorder.
The group size in the present study provided power of 0.78 to detect a medium effect (effect size=0.25). However, given the low preference ratings relative to the 200-mm visual analog scale, the group may not have been large enough to detect a small effect of paternal history on sweet preference. A recent study (14) examined taste responses to sweet, salty, bitter, and sour solutions among sons of male alcoholics. Although there was no evidence in that study of sweet preference as a marker for alcoholism risk, subjects with a paternal history of alcoholism rated lower concentrations of citric acid as more intense and higher concentrations of sodium chloride as more unpleasant than did subjects without a paternal history of alcoholism. These findings suggest that salt taste sensitivity or sour taste preference may be useful markers of alcoholism risk.
Although children of an alcoholic father are statistically at risk of developing alcohol dependence, the subjects included in the present study were selected for an absence of alcohol dependence. The average age of the group with a paternal history of alcoholism was >25 years, thereby virtually excluding persons with early-onset alcoholism, which may be the subtype most closely associated with a greater sweet preference (13). Although a prospective, longitudinal design would be most useful for determining whether sweet preference predisposes to the development of alcohol dependence, such a design would be expensive and time-consuming. Cross-sectional studies of the type reported here should include children or adolescents to determine whether the sucrose preference observed among alcoholics (11, 13) is a predictor of early-onset alcohol dependence or a consequence of heavy drinking.
 |
Received May 16, 2000; revision received Sept. 26, 2000; accepted Dec. 1, 2000. From the Alcohol Research Center, Department of Psychiatry, University of Connecticut School of Medicine. Address reprint requests to Dr. Kranzler, Department of Psychiatry, University of Connecticut Health Center, Farmington, CT 06030-2103; [email protected] (e-mail). Supported by NIH grants AA-03510 and AA-00239 from the National Institute on Alcohol Abuse and Alcoholism, RR-06192 (General Clinical Research Center), and the Dental Clinical Research Center at the University of Connecticut Health Center. The authors thank Lynn McLaughlin, R.N., for help in conducting the study.
1. Gelernter J: Genetic factors in alcoholism: evidence and implications, in The Pharmacology of Alcohol Abuse. Edited by Kranzler HR. New York, Springer-Verlag, 1995, pp 297–313Google Scholar
2. Swinson, RP: Genetic markers and alcoholism. Recent Dev Alcohol 1983; 1:9–21Crossref, Medline, Google Scholar
3. Ramirez I, Fuller JL: Genetic influence on water and sweetened water consumption in mice. Physiol Behav 1976; 16:163–168Crossref, Medline, Google Scholar
4. Kampov-Polevoy AB, Kasheffskaya OP, Sinclair JD: Initial acceptance of ethanol: gustatory factors and patterns of alcohol drinking. Alcohol 1990; 7:83–85Crossref, Medline, Google Scholar
5. Sinclair JD, Kampov-Polevoy A, Stewart R, Li TK: Taste preferences in rat lines selected for low and high alcohol consumption. Alcohol 1992; 9:155–160Crossref, Medline, Google Scholar
6. Overstreet DH, Kampov-Polevoy AB, Rezvani AH, Murrelle L, Halikas JA, Janowsky DS: Saccharin intake predicts ethanol intake in genetically heterogeneous rats as well as different rat strains. Alcohol Clin Exp Res 1993; 17:366–369Crossref, Medline, Google Scholar
7. Stewart RB, Murphy L, Lumeng L, Li TK: Sweet preference and spontaneous motor activity are correlated with alcohol intake in the F2 progeny of alcohol-preferring (P) and non-preferring (NP) rat cross (abstract). Alcohol Clin Exp Res 1997; 21(suppl):16Google Scholar
8. Kampov-Polevoy AB, Garbutt JC, Janowsky D: Association between preference for sweets and excessive alcohol intake: a review of animal and human studies. Alcohol Alcohol 1999; 34:386–395Crossref, Medline, Google Scholar
9. Phillips TJ, Crabbe JC, Metten P, Belknap JK: Localization of genes affecting alcohol drinking in mice. Alcohol Clin Exp Res 1994; 18:931–941Crossref, Medline, Google Scholar
10. Greene LS, Desor JA, Maller O: Heredity and experience: their relative importance in the development of taste preference in man. J Comp Physiol Psychol 1975; 89:279–284Crossref, Medline, Google Scholar
11. Kampov-Polevoy A, Garbutt JC, Janowsky D: Evidence of preference for a high-concentration sucrose solution in alcoholic men. Am J Psychiatry 1997; 154:269–270Link, Google Scholar
12. Spitzer RL, Williams JBW: Structured Clinical Interview for DSM-III-R (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1985Google Scholar
13. Kampov-Polevoy AB, Garbutt JC, Davis CE, Janowsky DS: Preference for higher sugar concentrations and Tridimensional Personality Questionnaire scores in alcoholic and nonalcoholic men. Alcohol Clin Exp Res 1998; 19:610–614Crossref, Google Scholar
14. Scinska A, Bogucka-Bonikowska A, Koros E, Polanowska E, Habrat B, Kukwa A, Kostowski W, Bienkowski P: Taste responses in sons of male alcoholics. Alcohol Alcohol 2001; 36:79–84Crossref, Medline, Google Scholar



