A Novel Augmentation Strategy for Treating Resistant Major Depression
Abstract
OBJECTIVE: Treatment-resistant depression is a significant public health concern; drug switching or augmentation often produce limited results. The authors hypothesized that fluoxetine could be augmented with olanzapine to successfully treat resistant depression. METHOD: An 8-week double-blind study was conducted with 28 patients who were diagnosed with recurrent, nonbipolar, treatment-resistant depression without psychotic features. Subjects were randomly assigned to one of three groups: olanzapine plus placebo, fluoxetine plus placebo, or olanzapine plus fluoxetine. RESULTS: Fluoxetine monotherapy produced minimal improvement on various scales that rate severity of depression. The benefits of olanzapine monotherapy were modest. Olanzapine plus fluoxetine produced significantly greater improvement than either monotherapy on one measure and significantly greater improvement than olanzapine monotherapy on the other measures after 1 week. There were no significant differences between treatment groups on extrapyramidal measures nor significant adverse drug interactions. CONCLUSIONS: Olanzapine plus fluoxetine demonstrated superior efficacy for treating resistant depression compared to either agent alone.
Up to 30% of patients with major depression fail to respond to conventional treatments (1–4). Antipsychotic agents may exhibit antidepressant activity, either alone or in combination with an antidepressant (5), particularly in depression with psychotic features (6–9). However, wide-scale application of augmentation with typical antipsychotics has been largely precluded by the high risk of extrapyramidal symptoms and/or tardive dyskinesia (10, 11). In contrast, novel antipsychotic agents such as olanzapine exhibit a substantially lower risk of extrapyramidal symptoms (12) and tardive dyskinesia (13).
To our knowledge, there are no controlled trials of atypical antipsychotics in patients with treatment-resistant, nonpsychotic unipolar depression. We conducted a randomized double-blind trial to assess the efficacy and safety of olanzapine combined with the selective serotonin reuptake inhibitor (SSRI) fluoxetine versus either agent alone in subjects diagnosed with recurrent major depressive disorder (nonbipolar) without psychotic features who were unresponsive to conventional antidepressant therapy.
Method
Written informed consent was obtained from all participants. The study was conducted between April 1997 and June 1998 in outpatients who met DSM-IV criteria for recurrent major depression without psychotic features and were resistant to conventional antidepressant pharmacotherapy. Patients with a history of psychosis, dysthymic disorder, or bipolar disorder were excluded. Treatment resistance was defined retrospectively by history of failure to respond to antidepressants of two different classes, one of which was not an SSRI, after at least 4 weeks of therapy at an acceptable therapeutic dose. Failure to respond was confirmed prospectively during a screening period in which fluoxetine was given. At entry, patients were required to score ≥20 on the 21-item Hamilton Depression Rating Scale (14).
The investigation consisted of three phases. The first was a 6-week open-label screening phase in which fluoxetine was given in escalating doses. Patients were titrated from an initial fluoxetine dose of 20 mg/day to the maximum tolerable dose, up to 60 mg/day. The second phase was an 8-week double-blind trial in which nonresponders to fluoxetine were randomly assigned in a 1:1:1 ratio to receive olanzapine plus placebo (“olanzapine”), fluoxetine plus placebo (“fluoxetine”), or olanzapine plus fluoxetine (“combination”). Patients in the olanzapine group discontinued fluoxetine on the day of random assignment. For all other patients, the fluoxetine dose received 1 week before random assignment remained unchanged throughout the double-blind period. For patients receiving olanzapine, the initial dose was 5 mg/day, titrated weekly within a range of 5–20 mg/day on the basis of response and tolerability.
The final study period was an 8-week open-label extension of olanzapine plus fluoxetine therapy. Only patients who successfully completed the double-blind phase were eligible to enter the open-label phase. Initial doses and subsequent titrations of olanzapine and fluoxetine were identical to those in the acute phase and were made at the investigators’ discretion. Analyses were performed on an intent-to-treat basis (15). Repeated measures analysis of variance was used to assess mean change from baseline, while the last observation carried forward was used to analyze weekly visits. All significance tests were performed at a two-tailed alpha level of 0.016, incorporating a Bonferroni correction for multiple comparisons.
Results
Thirty-four patients entered the open-label screening period with fluoxetine treatment. One subject responded during the screening; five others dropped out during the study (three for protocol violations and one for lack of efficacy; one was lost to follow-up). There were no significant differences between the three treatment groups on baseline depression ratings or demographic characteristics (age, gender, or ethnic origin) before random assignment. The majority of the 28 patients randomly assigned to double-blind therapy were women (75%) and white (96%). The mean age was 42 years (SD=11).
During double-blind therapy, the mean modal dose of fluoxetine was 52.0 mg/day for both the monotherapy and combination groups (SD=14.0 and SD=10.3, respectively). The mean modal dose of olanzapine was 12.5 mg/day (SD=5.3) and 13.5 mg/day (SD=4.1) for the monotherapy and combination groups, respectively.
The combination group (N=10) achieved greater improvement from baseline on the Montgomery-Åsberg Depression Rating Scale (16) than either monotherapy group (combination, –13.6) (olanzapine, –2.8; pair-wise F=2.22, df=8, 176, p=0.03) (fluoxetine, –1.2; pair-wise F=2.78, df=8, 176, p=0.006) on the basis of a repeated measures analysis with independent variables of therapy (F=1.46, df=2, 25, p=0.25), time (F=9.55, df=8, 176, p=0.001), and therapy-by-time (F=2.41, df=16, 176, p=0.003). Improvement was also greater with olanzapine plus fluoxetine on the Hamilton depression scale total score (14) than with olanzapine monotherapy but not significantly greater than with fluoxetine monotherapy (combination, –11.7) (olanzapine, –5.9; pair-wise F=2.23, df=8, 176, p=0.03) (fluoxetine, –3.8; pair-wise F=1.87, df=8, 176, p=0.07) on the basis of a repeated measures analysis with independent variables of therapy (F=1.52, df=2, 25, p=0.24), time (F=13.60, df=8, 176, p=0.001), and therapy-by-time (F=2.26, df=16, 176, p=0.005).
Finally, the combination group achieved significantly greater improvement from baseline than the olanzapine monotherapy group, but not the fluoxetine group, on the severity of depression subscale of the CGI (17) (combination, –2.0) (olanzapine, –0.0; pair-wise F=2.63, df=8, 174, p=0.01) (fluoxetine, –0.4; pair-wise F=0.94, df=8, 174, p=0.48) on the basis of a repeated measures analysis with the independent variables of therapy (F=3.72, df=2, 25, p=0.04), time (F=3.29, df=8, 174, p=0.002), and therapy-by-time (F=1.74, df=16, 174, p=0.04).
The proportion of patients noted as responding (≥50% improvement) on the Montgomery-Åsberg Depression Rating Scale was significantly greater for the combination group (N=6, 60%; global Fisher’s p=0.007) than for the olanzapine group (N=0, 0%; pair-wise, Bonferroni-adjusted Fisher’s p=0.03) but not for the fluoxetine group (N=1, 10%; pair-wise, Bonferroni-adjusted Fisher’s p=0.11). Significant last-observation-carried-forward differences between the combination and the fluoxetine monotherapy groups were evident by week 1 of double-blind therapy on all three scales (Figure 1). The significant response of the combination group (N=9) was maintained throughout the 8-week open-label extension period on all three measures. However, patients receiving monotherapy (olanzapine=6, fluoxetine=7) during the double-blind phase did not improve significantly during open-label combination treatment.
Both drugs were well tolerated either alone or in combination. During double-blind therapy, one patient in the olanzapine group discontinued treatment because of an adverse event (ataxia). Completion rates were high (combination, N=9, 90%; fluoxetine, N=7, 70%; olanzapine, N=6, 75%). The most frequently reported significant adverse events included somnolence, increased appetite, asthenia, weight gain, headache, dry mouth, and nervousness. Of these, both increased appetite and weight gain occurred significantly more frequently among patients treated with olanzapine (both as monotherapy and in combination). No clinically significant changes in vital signs or laboratory analytes were found among treatment groups, nor were there significant differences in the incidence of extrapyramidal symptoms. Mean weight increases from baseline to endpoint were 0.88 kg (SD=1.33; N=10, p=0.06, Wilcoxon’s signed rank test), 6.07 kg (SD=2.57; N=8, p=0.008, Wilcoxon’s signed rank test), and 6.67 kg (SD=4.54; N=10, p=0.002, Wilcoxon’s signed rank test) for the fluoxetine, olanzapine, and combination groups, respectively. A total of 27 (96.4%) of the patients completed the open-label extension phase of the trial. One patient dropped out of the study because of fever secondary to infection.
Discussion
In the present study, the combination of olanzapine with fluoxetine in patients with treatment-resistant, nonpsychotic, unipolar depression produced superior improvements over either agent alone across a variety of measures. Clinical responses were evident by the first week, suggesting rapid onset of action. Overall, the three treatments were well tolerated. For example, 9 (90%) of the patients receiving olanzapine plus fluoxetine completed double-blind therapy. The rates of extrapyramidal symptoms did not differ significantly between treatment groups. Previous long-term observations of olanzapine treatment suggest a significantly lower risk of tardive dyskinesia than with haloperidol (13). Although the observation period in the present study was short, the absence of acute extrapyramidal symptoms (which may predict a risk for subsequent tardive dyskinesia) was encouraging. In this study, one treatment-emergent event among patients treated with olanzapine, both in monotherapy and in combination with fluoxetine, was weight gain, averaging more than 6 kg over the double-blind treatment period. Combined olanzapine and fluoxetine appears to be an effective and well-tolerated treatment for treatment-resistant depression.
In contrast with the significant response observed with the combined therapy, neither fluoxetine nor olanzapine alone was effective in this resistant population. It therefore appears that neither the serotonin reuptake blockade of fluoxetine nor the pleiotropic receptor effects of olanzapine (18, 19) individually were beneficial in treating resistant depression. Concomitant administration of fluoxetine and olanzapine results in a small increase in olanzapine maximum concentration and area under the curve, zero to infinity, and a small decrease in olanzapine plasma clearance (unpublished work by Gossen et al.). Such changes, although statistically significant, are small in comparison to the overall variability between individuals and are unlikely to result in a clinically significant pharmacokinetic interaction.
Alternatively, combined administration likely introduces a pharmacodynamic synergy. Zhang and colleagues (20) demonstrated that 3 hours after administration of olanzapine, norepinephrine and dopamine concentrations in the rat prefrontal cortex returned to baseline values. With fluoxetine treatment, norepinephrine and dopamine levels increase to 188% and 143% of baseline values, respectively. However, when both drugs are given, norepinephrine and dopamine levels increase to 269% and 349% of baseline values, respectively. This suggests a neurochemical basis for the synergistic antidepressant effect observed in the present trial. The prefrontal cortex is rich in mesocorticolimbic dopamine innervation (21–23). Coupled with the well-chronicled role of norepinephrine in mood states (24), this suggests potential targets for treating resistant depression.
The antidepressant efficacy observed in the current study with the combined administration of olanzapine and fluoxetine is intriguing. The robust effect size and rapid onset of action suggest promise for this approach. Because of the small group size, these results should be considered preliminary. Nevertheless, considering the public health impact of treatment-resistant depression, these results and future treatment alternatives should be welcomed. To this end, we are currently conducting studies to investigate further the efficacy and safety of combined olanzapine and fluoxetine in the treatment of patients with refractory depression.
Received July 23, 1999; revisions received Feb. 25 and June 22, 2000; accepted July 17, 2000. From the Department of Psychiatry, Vanderbilt University Medical Center; Lilly Research Laboratories, Indianapolis; the Department of Psychiatry, McLean Hospital, Belmont, Mass.; and the Clinical Neuroscience Research Institute, San Diego. Address reprint requests to Dr. Shelton, Department of Psychiatry, Vanderbilt University Medical Center, 1500 21st Ave. South, Nashville, TN 37212; [email protected] (e-mail). Supported in part by Eli Lilly and Company and by NIMH grant MH-01741. Dr. Shelton is supported by an NIMH Research Career Award (MH-01741). The authors thank Ms. Linda Todd for assistance with the clinical study and Mr. Scott Andersen for statistical assistance.
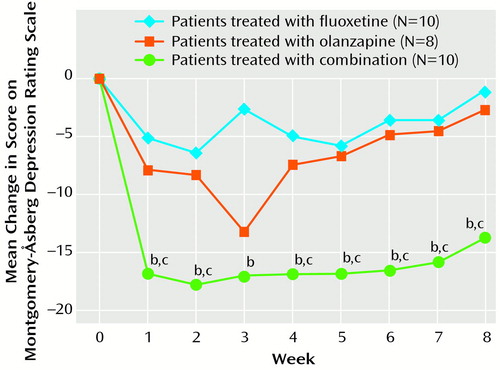
Figure 1. Weekly Change From Baseline in Response Rate (last observation carried forward) for Patients Treated With Fluoxetine, Olanzapine, or a Combination of Botha
aCombination superior to fluoxetine or olanzapine (p<0.05, repeated measures analysis of variance).
bSignificantly superior to fluoxetine (p<0.05, t test with Bonferroni correction).
cSignificantly superior to olanzapine (p<0.05, t test with Bonferroni correction).
1. Amsterdam JD, Hornig-Rohan M: Treatment algorithms in treatment-resistant depression. Psychiatr Clin North Am 1996; 19:371–386Crossref, Medline, Google Scholar
2. Fawcett J: Progress in treatment-resistant and treatment-refractory depression: we still have a long way to go. J Clin Psychiatry 1994; 24:214–216Google Scholar
3. Nierenberg AA, Amsterdam JD: Treatment-resistant depression: definition and treatment approaches. J Clin Psychiatry 1990; 51(suppl 6):39–47Google Scholar
4. Roose SP, Glassman AH, Walsh BT, Woodring S: Tricyclic nonresponders: phenomenology and treatment. Am J Psychiatry 1986; 143:345–348Link, Google Scholar
5. Robertson MM, Trimble MR: Major tranquillisers used as antidepressants: a review. J Affect Disord 1982; 4:173–193Crossref, Medline, Google Scholar
6. Rothschild AJ, Samson JA, Bessette MP, Carter-Campbell JT: Efficacy of the combination of fluoxetine and perphenazine in the treatment of psychotic depression. J Clin Psychiatry 1993; 54:338–342Medline, Google Scholar
7. Spiker DG, Weiss JC, Dealy RS, Griffin SJ, Hanin I, Neil NF, Perel JM, Rossi AJ, Soloff PH: The pharmacological treatment of delusional depression. Am J Psychiatry 1985; 142:430–436Link, Google Scholar
8. Wolfersdorf M, Barg T, Konig F, Leibfarth M, Grunewald I: Paroxetine as antidepressant in combined antidepressant-neuroleptic therapy in delusional depression: observation of clinical use. Pharmacopsychiatry 1995; 28:56–60Crossref, Medline, Google Scholar
9. Wolfersdorf M, Konig F, Straub R: Pharmacotherapy of delusional depression: experience with combinations of antidepressants with the neuroleptics zotepine and haloperidol. Neuropsychobiology 1994; 29:189–193Crossref, Medline, Google Scholar
10. Casey DE: Neuroleptic-induced acute extrapyramidal syndromes and tardive dyskinesia. Psychiatr Clin North Am 1993; 16:589–610Crossref, Medline, Google Scholar
11. Jeste DV, Caligiuri MP: Tardive dyskinesia. Schizophr Bull 1993; 19:303–315Crossref, Medline, Google Scholar
12. Tran PV, Dellva MA, Tollefson GD, Beasley CM Jr, Potvin JH, Kiesler GM: Extrapyramidal symptoms and tolerability of olanzapine versus haloperidol in the acute treatment of schizophrenia. J Clin Psychiatry 1997; 58:205–211Crossref, Medline, Google Scholar
13. Tollefson GD, Beasley CM Jr, Tamura RN, Tran PV, Potvin JH: Blind, controlled, long-term study of the comparative incidence of treatment-emergent tardive dyskinesia with olanzapine or haloperidol. Am J Psychiatry 1997; 154:1248–1254Google Scholar
14. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
15. Gillings D, Koch G: The application of the principle of intention-to-treat to the analysis of clinical trials. Drug Info J 1991; 25:411–425Crossref, Google Scholar
16. Montgomery SA, Åsberg M: A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Crossref, Medline, Google Scholar
17. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 218–222Google Scholar
18. Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, Seeman P, Wong TD: Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 1996; 14:87–96Crossref, Medline, Google Scholar
19. Bymaster FP, Rasmussen K, Calligaro DO, Nelson DL, DeLapp NW, Wong DT, Moore NA: In vitro and in vivo biochemistry of olanzapine: a novel, atypical antipsychotic drug. J Clin Psychiatry 1997; 58(suppl 10):28–36Google Scholar
20. Zhang W, Perry WK, Wong DT, Potts BD, Bao J, Tollefson GD, Bymaster FP: Synergistic effects of olanzapine and other antipsychotic agents in combination with fluoxetine on norepinephrine and dopamine release in rat prefrontal cortex. Neuropsychopharmacology 2000; 23:250–262Crossref, Medline, Google Scholar
21. Deutch AY: The regulation of subcortical dopamine systems by the prefrontal cortex: interactions of central dopamine systems and the pathogenesis of schizophrenia. J Neural Transm 1992; 36(suppl):61–89Google Scholar
22. Knable MB, Weinberger DR: Dopamine, the prefrontal cortex and schizophrenia. J Psychopharmacol 1997; 11:123–131Crossref, Medline, Google Scholar
23. Willner P: Dopaminergic mechanisms in depression and mania, in Psychopharmacology: The Fourth Generation of Progress. Edited by Bloom FE, Kupfer DJ. New York, Raven Press, 1995, pp 921–931Google Scholar
24. Schatzberg AF, Schildkraut JJ: Recent studies on norepinephrine systems in mood disorders. Ibid, pp 911–920Google Scholar



