Central Auditory Processing in Patients With Auditory Hallucinations
Abstract
OBJECTIVE: Data from a full assessment of auditory perception in patients with schizophrenia were used to investigate whether auditory hallucinations are associated with abnormality of central auditory processing. METHOD: Three groups of subjects participated in auditory assessments: 22 patients with psychosis and a recent history of auditory hallucinations, 16 patients with psychosis but no history of auditory hallucinations, and 22 normal subjects. Nine auditory assessments, including auditory brainstem response, monotic and dichotic speech perception tests, and nonspeech perceptual tests, were performed. Statistical analyses for group differences were performed using analysis of variance and Kruskal-Wallis tests. The results of individual patients with test scores in the severely abnormal range (more than three standard deviations from the mean for the normal subjects) were examined for patterns that suggested sites of dysfunction in the central auditory system. RESULTS: The results showed significant individual variability among the subjects in both patient groups. There were no group differences on tests that are sensitive to low brainstem function. Both patient groups performed poorly in tests that are sensitive to cortical or high brainstem function, and hallucinating patients differed from nonhallucinating patients in scores on tests of filtered speech perception and response bias patterns on dichotic speech tests. Six patients in the hallucinating group had scores in the severely abnormal range on more than one test. CONCLUSIONS: Hallucinations may be associated with auditory dysfunction in the right hemisphere or in the interhemispheric pathways. However, comparison of results for the patient groups suggests that the deficits seen in hallucinating patients may represent a greater degree of the same types of deficits seen in nonhallucinating patients.
Auditory hallucinations represent a common, often disabling symptom in schizophrenia. Their auditory nature raises the possibility of associated auditory processing deficits. Previous auditory studies involving patients with schizophrenia have investigated hypotheses relating to hemisphere asymmetry by using dichotic listening tasks. The results of these studies have been variable. Several studies (1–4) that have used identical dichotic speech tests (nonsense syllables and words) have found reduced right ear advantage in patients with schizophrenia compared to normal subjects. On the other hand, two studies involving a task that required subjects to respond to three or four dichotic digit pairs presented in quick succession showed an enhanced right ear advantage in patients with schizophrenia compared to normal subjects (5, 6). A reduced right ear advantage for patients with schizophrenia has been shown by using a dichotic consonant-vowel test (7), whereas no abnormal ear asymmetry was found by using the staggered spondaic word test (8). A study that used a dichotic monitoring task, in which patients pushed a button when they heard a target word, showed a significantly increased right ear advantage for subjects experiencing acute psychotic episodes, compared to normal subjects (9).
Variability in the findings of auditory dichotic tests is likely to arise from heterogeneity in the pathological processes and in the types and intensity of symptoms in the patient populations and from differences in the perceptual tasks required by the various tests. The idea that right ear advantage is a simple measure of laterality of function is questionable when the subjects who are tested may have pathological processes affecting the central auditory processing of information.
Few studies have examined the relationship between hallucinations and the results of auditory tests. Two studies that used the same test have found that hallucinations were negatively correlated with right ear advantage in patients with schizophrenia who had hallucinations (4, 10). Hoffman et al. (11) found that hallucinating patients performed more poorly than nonhallucinating patients on masked speech tracking and sentence repetition tasks, suggesting that hallucinations are associated with disrupted speech perception and verbal working memory.
The purpose of the study reported here was to investigate the hypothesis that individuals who experience auditory hallucinations have an associated abnormality of central auditory processing. Many studies have investigated a particular aspect of auditory perception in patients with psychosis. However, we are not aware of any studies that have been based on a comprehensive evaluation of such patients that has included tests with previously documented results linked to known lesions of the central auditory system. We designed the study reported here to investigate a broad range of auditory perception abilities, and we included audiological tests that are widely used clinically for the diagnosis of central auditory pathology. The results of the study will be used to formulate more specific hypotheses about sites of dysfunction and functional abnormalities that will be tested in future studies, as well as to formulate hypotheses relevant to improvement of the clinical management of the symptom of hallucination.
Method
Subjects
Three subject groups were included in the study (Table 1). The normal comparison group (N=22) were volunteers with no personal or family history of psychiatric illness or hallucinations. The currently hallucinating (N=22) and nonhallucinating (N=16) patient groups were recruited from outpatient clinics. Their psychiatric diagnosis was established on the basis of patient interviews by using the Structured Clinical Interview for DSM-III-R (SCID) (12). The frequency and phenomenology of auditory hallucinations in the hallucinating patients were established by using the Mental Health Research Institute Unusual Perception Scale (13). The SCID and Mental Health Research Institute Unusual Perception Scale procedures have both been shown to have high reliability (13, 14).
Subjects were designated as hallucinating patients if they had reported hearing voices during the week before testing. The frequency of hallucinations during that week was 1–5 times for two patients and 6–20 times for 17 patients; three patients reported constant hallucinations. Subjects were included in the psychotic comparison group if they had a diagnosis of a psychiatric illness but reported that they had never experienced auditory hallucinations.
Seventeen of the 22 patients in the hallucinating group had a diagnosis of chronic schizophrenia (13 with the paranoid subtype, two with the undifferentiated subtype, one with the disorganized subtype, and one for whom the subtype was not reported). The remaining five patients in the group had schizoaffective disorder. Eleven of the 16 patients in the nonhallucinating group had a diagnosis of chronic schizophrenia (five with the paranoid subtype, four with the undifferentiated subtype, and two for whom the subtype was not reported). One patient in that group had schizoaffective disorder, one had schizophreniform disorder, and three had bipolar disorder.
All subjects were experienced English speakers. Subjects were excluded if they had a history of neurological insult or illness, epilepsy, recent drug abuse, or drug-induced hallucinations. Before subjects participated in the study, measurements of their hearing threshold and acoustic immittance were obtained. Subjects with hearing abnormalities (having an average hearing threshold at 500, 1000, and 2000 Hz greater than 20 dB HL) or a significant middle ear disorder were excluded. After subjects were informed of the aims and procedures of the research and given a statement describing the aims and procedures in plain English, they gave signed consent to participate.
Procedures
All perceptual tests were carried out in a sound-treated room within one session. Auditory brainstem responses were measured in a subsequent session at a time up to 2 months after the perceptual tests. Patients were requested to signal any periods of hallucinations during the testing. The parts of the tests administered during those periods were repeated, and the data from the nonhallucinatory periods were used in the analysis. In all perceptual tests, the signal was delivered by using a Madsen OB822 clinical audiometer through TDH-39 headphones at a 50 dB sensation level. Some subjects did not complete all the tests for practical reasons (the N values for each test are listed in Table 2).
The test battery consisted of nine standard audiological diagnostic procedures.
Auditory brainstem response
This response is the auditory evoked potential occurring in the first 10 msec after presentation of the stimulus; it is sensitive to VIII nerve and low brainstem lesions (15). Ipsilateral responses were recorded with stimulus rates of both 12 clicks/sec and 50 clicks/sec, and contralateral responses were recorded with a stimulus rate of 12 clicks/sec. Responses to 2,000 stimuli (100-msec clicks) were averaged in two trials for each condition. The latencies and interpeak intervals of waves I, II, III, and V were measured, interaural differences in these measures were calculated, and the wave morphology was examined. Because established normal values for auditory brainstem response were available, this test was administered to only the two patient groups.
Monaural filtered speech
This test uses monaurally presented AB words (16), filtered into two bands (360–890 Hz and 1750–2220 Hz) and presented simultaneously to one ear. It is sensitive to cortical and high brainstem lesions (17).
Binaural fusion
This test uses filtered speech material, but the speech is presented dichotically (one bandpass segment in each ear). The results are compared with those of the monaural filtered speech test above. A significant monaural enhancement (where the dichotic score is significantly less than either monaural score) is indicative of lower brainstem disorders (18).
Rapidly alternating speech perception
This test consists of 20 sentences during which the signal is alternated between ears in a 600-msec cycle. The test is sensitive to low brainstem disorders (19).
Monaural speech discrimination in ipsilateral competition
These two tests consist of monaural speech discrimination of words in ipsilateral white noise (signal-to-noise ratio of +15 dB) and competing verbal messages (signal-to-noise ratio of +5 dB). The tests are sensitive to temporal lobe and brainstem lesions (20).
Monaural frequency tone patterns
This test consists of three tones with patterns of low (880 Hz) and high (1122 Hz) frequencies. The subject must identify the pattern of pitch change with a verbal or hummed response. The test is sensitive to cortical lesions (21) and deficits in interhemispheric transfer (22). As verbal responses necessitate transfer of information between hemispheres, abnormal differences between verbal and hummed responses have diagnostic significance.
Staggered spondaic word test
This dichotic test consists of spondees presented to each ear such that the last half of the first spondee and the first half of the second spondee are simultaneous. The word errors are scored in four conditions; left and right ear in competing and noncompeting modes (where competing modes refer to the simultaneous word parts). The scores are further analyzed for “order” and “ear” effects (significantly more errors on the first or second spondee, or when the left or right ear leads, respectively) and the number of reversals (incorrect order of word segments). The test is sensitive to temporal lobe lesions and deficits in the interhemispheric pathways (23).
Competing environmental sounds
This test consists of 20 pairs of environmental sounds presented dichotically. The subject must respond by pointing to two pictures from a closed set that they feel best represent the sounds heard. The test is sensitive to cortical lesions and is interpreted in relation to the staggered spondaic word test (23).
Dichotic consonant-vowels
This test consists of nonidentical pairs of consonants-vowels (e.g., “pa,” “ba”) presented dichotically. The subject chooses (from a closed set of six) which two consonant-vowels were presented. Practice items are followed by one list (50 pairs) presented simultaneously to each ear and then by two lists presented with the stimulus to one ear (left then right) leading the stimulus to the other ear by 90 msec. The test is also analyzed for “lag effect,” in which the lagging ear in the lag condition should perform better than the same ear in the simultaneous condition. This test is sensitive to cortical and hemispheric transfer lesions (24).
Statistical Analysis
Where scores were normally distributed, differences between subject groups were analyzed using analysis of variance (ANOVA), followed by Tukey’s pairwise comparisons. Otherwise, Kruskal-Wallis tests followed by Mann-Whitney tests (with Bonferroni correction) were used. Paired t tests or Wilcoxon signed ranks tests were used to examine right ear/left ear differences or response biases in each group as appropriate. A significance level of p<0.05 was used for the ANOVA and Kruskal-Wallis tests, and a family error rate of p<0.05 was used for the post hoc tests for any one assessment. Because a number of tests were performed, the risk of type I error (chance significance) was increased. The possible influence of the increased risk on the interpretation of findings is addressed in the discussion.
Results
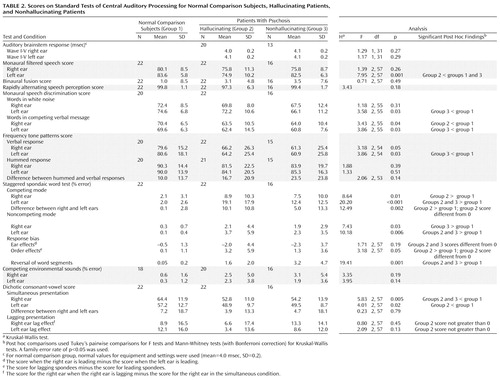
The subjects’ mean scores on the test battery and results of the statistical analyses of the scores are summarized in Table 2. Instances in which the mean test score was different from an expected 0 (response biases on the staggered spondaic word test) or not greater than 0, as expected (lag effect on the dichotic consonant-vowel test), are also noted.
Both patient groups and the normal comparison group performed similarly on many tests, including all of the tests that reflected lower brainstem function (rapidly alternating speech perception, binaural fusion, and auditory brainstem response). On most tests that were sensitive to dysfunction more central than the lower brainstem, either or both patient groups performed significantly poorer than the normal group, but not significantly different from each other. These included monaural speech in ipsilateral competition (left ear for speech in white noise and both ears for speech in competing message), monaural frequency tone patterns in the left ear with verbal response, performance levels on the two dichotic speech tests (staggered spondaic words and dichotic consonant-vowels), and leading-ear and reversal response biases on the staggered spondaic word test.
Only one test (monaural filtered speech in the left ear) yielded a significant difference in absolute performance level between the hallucinating and nonhallucinating groups (t=2.84, df=35, p<0.007). These data were further analyzed to test for possible confounding variables. To assess whether medication level (chlorpromazine equivalent dosage of antipsychotic medication), age, years of education, handedness, or sex influenced the overall performance, we constructed two general linear models, one that included the three subject groups with age and education as covariates and sex and handedness as factors and one that included the two patient groups with medication as a covariate. No significant effects of these factors (or interaction terms) were found.
Three response patterns in the dichotic tests were significantly different from the expected pattern only for the hallucinating group: the staggered spondaic word test showed a significant right ear advantage (Wilcoxon signed ranks test, T=3.0, p=0.001, N=16) and a significant word order response favoring the second spondee (Wilcoxon signed ranks test, T=156.5, p=0.01, N=19); the dichotic consonant-vowel test showed an absence of the expected lag effect in both ears (t=1.78, df=21, p=0.09 for the right ear; t=1.16, df=21, p=0.26 for the left ear).
Results for individual subjects were studied to determine their pattern of abnormal scores on the tests. Abnormal scores and severely abnormal scores were those that were different from the mean score for the normal comparison subjects by two or more or by three or more standard deviations, respectively. None of the normal comparison subjects, 16 hallucinating subjects (73%), and 10 nonhallucinating subjects (63%) had abnormal scores on more than one test. The overall patterns of abnormal scores in the two patient groups were similar and were consistent with the poorer overall performance of both patient groups in contrast to the normal comparison group on many tests (Table 2). Abnormal scores on the test of monaural speech discrimination in ipsilateral competition (using competing message) were the most common in both groups, followed by abnormal scores on the dichotic speech tests (staggered spondaic words and dichotic consonant-vowels) and left ear verbal response on the frequency tone patterns test. The proportions of subjects in the two groups with two or more abnormal scores were not significantly different.
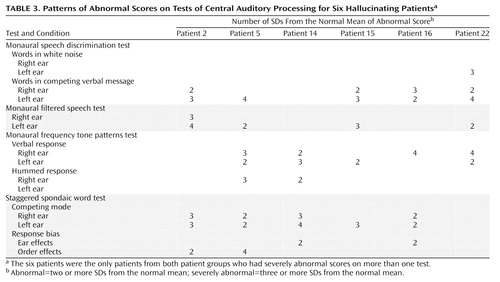
None of the normal subjects had severely abnormal scores on any tests, and none of the nonhallucinating group had scores in the severely abnormal range on more than one test. Six hallucinating patients, however, had severely abnormal scores on at least two tests (four of the six subjects had severely abnormal scores on three tests). All of these six subjects were right-handed, and three were female. The six subjects did not differ significantly from the rest of the hallucinating group (or from the nonhallucinating group) in duration of illness (mean=9.8 years, SD=7.6, range=1–23 years) or medication level (mean dose=717 mg/day chlorpromazine equivalants, SD=396, range=200–1350 mg/day chlorpromazine equivalants). The pattern of severely abnormal test scores for these subjects is detailed in Table 3.
Discussion
The analysis of group differences in central auditory processing supported previous studies in which abnormalities in auditory processing were associated with schizophrenia. Of the 30 group comparisons shown in Table 2, 15 showed significant group differences at the p<0.05 level. However, it is important to consider that factors such as poorer attention or motivation can contribute to a lower overall performance in both the patient groups. Thus, when using response deficits to deduce possible sites of dysfunction, more importance should be placed on the pattern of deficiency across different conditions and tests within the same subject group. For example, although both patient groups had deficits in all four conditions of the staggered spondaic word test, the deficits were more significant in the left ear, a pattern that is associated with dysfunction either in the right auditory cortex or in pathways connecting the two hemispheres (19, 22). Similarly, both patient groups showed a tendency for the largest decrement to occur in the verbal response modes of the frequency tone patterns test. This pattern may also indicate dysfunction of the pathways between the right hemisphere and language production areas of the cortex (22). This interpretation is supported by studies that have found abnormalities of the corpus callosum in patients with schizophrenia (25–29). In general, the tests showed no indication of lower brainstem dysfunction in either patient group, in contrast to the findings of Lindstrom et al. (30), who found abnormalities in the auditory brainstem response of hallucinating subjects, but not of nonhallucinating subjects.
Only the monaural filtered speech test for the left ear yielded a significant difference in absolute performance levels between the hallucinating and nonhallucinating groups (t=2.84, df=35, p<0.007). In view of the number of comparisons between the two groups, however, there is a significant chance (p=0.2) of a type I error for this significance level. A left ear deficit on this test has been associated with dysfunction in the right hemisphere (19) or in the corpus callosum (22, 31). Abnormality of filtered speech perception has also been noted in some patients with pathology in the high brainstem region (19). Although Early et al. (32) suggested that the right hemisphere may be inhibited by antipsychotic drugs, no significant correlation was found between the dose of medication and scores for monaural filtered speech in the left ear in the patient groups.
The significant response pattern abnormalities shown only by the hallucinating group on the dichotic speech tests (the staggered spondaic word test and the dichotic consonant-vowel test) should also be interpreted with caution in view of the nonsignificant group differences between the hallucinating subjects and the nonhallucinating subjects. These patterns may be characteristic of schizophrenia, but they may show a greater degree of dysfunction among hallucinating subjects. In the staggered spondaic word test, a significant error rate in either ear is considered to reflect a dysfunction in the auditory pathways, and asymmetry of error rate between the ears is interpreted in light of results for research subjects with known pathologies. The significant right ear advantage for the hallucinating group may reflect either right hemisphere dysfunction or a deficit in interhemispheric transfer (19, 22). A word order effect favoring the second spondee has been associated with lesions in the frontal and anterior temporal lobes, resulting in problems with expressive language, memory, or behavior (33). This effect is consistent with the findings of Hoffman et al. (11). Similarly, a lack of the expected lag effect on the dichotic consonant-vowel test has been associated with cortical or interhemispheric pathway dysfunction (24).
Both patient groups were heterogeneous in their ability to perform central auditory tests; some patients performed normally on all tests, and others performed abnormally on a number of tests. This variability, as well as the subtle nature of the group differences, implies that the dysfunction responsible for poor auditory processing skills in patients with schizophrenia, or in patients with hallucinations, is unlikely to be in the primary auditory areas of the cortex.
The results for the six hallucinating patients with severely abnormal scores on more than one test suggest interpretations that are broadly consistent with the tentative interpretations of the group differences. Five of the six had severely abnormal scores for the left ear in one or more monaural or dichotic speech tests. In these cases, it is unlikely that the site of dysfunction would be in the right primary auditory cortex, as none of these subjects had abnormal scores for the left ear on the dichotic consonant-vowel test. It is more likely that the site of dysfunction is distant from the primary auditory cortex, or that it is associated with deeper structures, affecting the high brainstem or interhemispheric pathways. A high brainstem dysfunction may explain the bilateral deficits of hallucinating subject 2. The possibility of deficits in interhemispheric transfer is further supported by the concurrent abnormalities on the frequency tone patterns test in these subjects. Four of the subjects had severely abnormal scores on one or more conditions of this test. In all cases a deficit was present in a verbal response mode. Because the specialized cortical areas for tone pattern and language-based responses are in opposite hemispheres, a good verbal response performance requires transfer of information between hemispheres. Hallucinating subject 5 also had a clear left ear advantage for both verbal and hummed responses (both right ear responses were severely abnormal), indicating a possible deficit in tone perception when this information must be transferred from the left to the right hemisphere.
It should be noted, however, that these patterns of deficit for the frequency tone patterns test were also present in the nonhallucinating patient group. In both patient groups, the mean scores were closest to the normal comparison mean score for the left ear hummed response condition (Table 2), in which there is theoretically no need for interhemispheric transfer of information. In addition, the left ear hummed response condition was the only condition for which no patient from either group had a severely abnormal score. The worst mean scores for both patient groups were for the two verbal response conditions, a result that is consistent with dysfunction in the interhemispheric pathways connecting the right and left hemispheres. Thus the deficits seen on this test may reflect the effects of the underlying mental illness, rather than an association with hallucinations per se.
Conclusions
This study has compared the central auditory processing of mentally ill patients with and without hallucinations. The results were characterized by wide variability among individuals in both patient groups. Some patients showed severe abnormalities of central auditory processing, and others performing normally on all tasks. On the majority of auditory tests, the performance of the hallucinating patients was not significantly different from that of the nonhallucinating patients. The overall pattern of abnormality in both patient groups was consistent with dysfunction of the right hemisphere (distant from the primary auditory cortex) and/or dysfunction of interhemispheric transfer pathways. The tests that showed differences between the hallucinating and nonhallucinating patients, and the patterns of abnormality for the six patients with the most severely abnormal scores (who were all hallucinating patients), were also consistent with dysfunction in the same areas. Involvement of deeper structures of the medial temporal lobe of either hemisphere, affecting intrahemispheric or interhemispheric transfer of information, cannot be ruled out. It is possible, therefore, that hallucinations are associated with a greater degree of the same type of auditory dysfunction that results from schizophrenia itself.
Further research is needed to investigate whether particular features or patterns of hallucinatory experience or the effectiveness of different strategies for coping with hallucinations are related to the presence of specific auditory processing abnormalities.
 |
 |
 |
Received May 3, 1999; revision received Nov. 29, 1999; accepted Dec. 1, 1999. From the Department of Otolaryngology and Human Communication Research Centre, University of Melbourne; and the Mental Health Research Institute of Victoria, Melbourne, Australia. Address reprint requests to Dr. McKay, Department of Otolaryngology, University of Melbourne, 384-388 Albert St., East Melbourne, 3002 Australia; [email protected] (e-mail). Funding for this study was provided by a grant from the National Health & Medical Research Council, the Human Communication Research Centre at the University of Melbourne, and the Mental Health Research Institute of Victoria. Ms. Headlam was supported by a scholarship from the Department of Human Services, Victoria, Australia. The authors thank Rosemary Thomas for interviewing and managing the patients, Andrew Mackinnon for statistical advice, and Graeme Clark for his advice.
1. Wexler BE, Giller EL Jr, Southwick S: Cerebral laterality, symptoms, and diagnosis in psychotic patients. Biol Psychiatry 1991; 29:103–116Crossref, Medline, Google Scholar
2. Ragland JD, Goldberg TE, Wexler BE, Gold JM, Torrey EF, Weinberger DR: Dichotic listening in monozygotic twins discordant and concordant for schizophrenia. Schizophr Res 1992; 7:177–183Crossref, Medline, Google Scholar
3. Grosh ES, Docherty NM, Wexler BE: Abnormal laterality in schizophrenics and their parents. Schizophr Res 1995; 14:155–160Crossref, Medline, Google Scholar
4. Bruder G, Rabinowicz E, Towey J, Brown A, Kaufmann CA, Amador X, Malaspina D, Gorman JM: Smaller right ear (left hemisphere) advantage for dichotic fused words in patients with schizophrenia. Am J Psychiatry 1995; 152:932–935Link, Google Scholar
5. Lishman WA, Toone BK, Colbourn CJ, McMeekan ERL, Mance RM: Dichotic listening in psychotic patients. Br J Psychiatry 1978; 132:333–341Crossref, Medline, Google Scholar
6. Lerner J, Nachson I, Carman A: Responses of paranoid and nonparanoid schizophrenics in a dichotic listening task. J Nerv Ment Dis 1977; 164:247–252Crossref, Medline, Google Scholar
7. Colbourn CJ, Lishman WA: Lateralization of function and psychotic illness: a left hemisphere deficit? in Hemisphere Asymmetries of Function in Psychopathology. Edited by Gruzelier J, Flor-Henry P. New York, Elsevier-North Holland, 1979, pp 539–559Google Scholar
8. Yozawitz A, Bruder G, Sutton S, Sharpe L, Gurland B, Fleiss J, Costa L: Dichotic perception: evidence for right hemisphere dysfunction in affective psychosis. Br J Psychiatry 1979; 135:224–237Crossref, Medline, Google Scholar
9. Wale J, Carr VJ: Dichotic listening asymmetries and psychotic symptoms in schizophrenia: a preliminary report. Psychiatry Res 1988; 25:31–39Crossref, Medline, Google Scholar
10. Green MF, Hugdahl K, Mitchell S: Dichotic listening during auditory hallucinations in patients with schizophrenia. Am J Psychiatry 1994; 151:357–362Link, Google Scholar
11. Hoffman RE, Rapaport J, Mazure CM, Quinlan DM: Selective speech perception alterations in schizophrenic patients reporting hallucinated “voices.” Am J Psychiatry 1999; 156:393–399Abstract, Google Scholar
12. Spitzer RL, Williams JBW: Structured Clinical Interview for DSM-III-R (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1987Google Scholar
13. Carter DM, Mackinnon A, Howard S, Zeegers T, Copolov DL: The development and reliability of the Mental Health Research Institute Unusual Perceptions Schedule (MUPS): an instrument to record auditory hallucinatory experience. Schizophr Res 1995; 16:157–165Crossref, Medline, Google Scholar
14. Segal DL, Hersen M, Van Hasselt VB: Reliability of the Structured Clinical Interview for DSM-III-R: an evaluative review. Compr Psychiatry 1994; 35:316–327Crossref, Medline, Google Scholar
15. Musiek FE, Gollegly KM, Kibbe KS, Verkest SB: Current concepts on the use of ABR and auditory psychophysical tests in the evaluation of brain stem lesions. Am J Otol 1988; 9(Dec suppl):25–34Google Scholar
16. Boothroyd A: Developments in speech audiometry. Sound 1968; 2:3–10Google Scholar
17. Musiek F, Baran J, Pinheiro M: Neuroaudiology: Case Studies. San Diego, Singular Publishing Group, 1994Google Scholar
18. Tobin H: Binaural interaction tasks, in Assessment of Central Auditory Dysfunction: Foundations and Clinical Correlates. Edited by Pinheiro M, Musiek F. Baltimore, Williams & Wilkins, 1985, pp 155–171Google Scholar
19. Lynn G, Gilroy J: Evaluation of central auditory dysfunction in patients with neurological disorders, in Central Auditory Dysfunction. Edited by Keith R. New York, Grune & Stratton, 1977, pp 177–221Google Scholar
20. Olsen W, Noffsinger D, Kurdziel S: Speech discrimination in quiet and in noise by patients with peripheral and central lesions. Acta Otolaryngol 1975; 80:375–382Crossref, Medline, Google Scholar
21. Musiek F, Pinheiro M: Frequency patterns in cochlear, brainstem and cerebral lesions. Audiology 1987; 26:79–88Crossref, Medline, Google Scholar
22. Musiek F, Kibbe K, Baran J: Neuroaudiological results from split-brain patients. Seminars in Hearing 1984; 5:219–229Crossref, Google Scholar
23. Katz J, Kusher D, Pack G: The use of competing speech (SSW) and environmental sounds (CES) for localizing brain lesions, in Central Auditory Assessment: The SSW Test, Development and Clinical Use. Edited by Arnst D, Katz J. London, College Hill Press, 1982, pp 227–236Google Scholar
24. Berlin C, Lowe-Bell S, Cullen J Jr, Thompson S, Loovis C: Dichotic speech perception: an interpretation of right-ear advantage and temporal offset effects. J Acoust Soc Am 1973; 53:699–709Crossref, Medline, Google Scholar
25. Raine A, Harrison G, Reynolds G, Sheard C, Cooper J, Medley I: Structural and functional characteristics of the corpus callosum in schizophrenics, psychiatric controls, and normal controls. Arch Gen Psychiatry 1990; 47:1060–1064Google Scholar
26. Swayze VW II, Andreasen NC, Ehrhardt JC, Yuh WT, Alliger RJ, Cohen GA: Developmental abnormalities of the corpus callosum in schizophrenia. Arch Neurol 1990; 47:805–808Crossref, Medline, Google Scholar
27. Woodruff P, McManus I, David A: Meta-analysis of corpus callosum size in schizophrenia. J Neurol Neurosurg Psychiatry 1995; 58:457–461Crossref, Medline, Google Scholar
28. David A, Wacharasindhu A, Lishman W: Severe psychiatric disturbance and abnormalities of the corpus callosum: review and case series. J Neurol Neurosurg Psychiatry 1993; 56:85–93Crossref, Medline, Google Scholar
29. Gunther W, Petsch R, Steinberg R, Moser E, Streck P, Heller H, Kurtz G, Hippius H: Brain dysfunction during motor activation and corpus callosum alterations in schizophrenia measured by cerebral blood flow and magnetic resonance imaging. Biol Psychiatry 1991; 29:535–555Crossref, Medline, Google Scholar
30. Lindstrom L, Klockhoff I, Svedberg A, Bergstrom K: Abnormal auditory brain-stem response in hallucinating schizophrenic patients. Br J Psychiatry 1987; 151:9–14Crossref, Medline, Google Scholar
31. Baran J, Musiek F, Reeves A: Central auditory function following anterior sectioning of the corpus callosum. Ear Hear 1986; 7:359–362Crossref, Medline, Google Scholar
32. Early TS, Haller JW, Posner MI, Raichle M: The left striato-pallidal hyperactivity model, in The Neuropsychology of Schizophrenia. Edited by David A, Cutting J. Hove, UK, Lawrence Erlbaum Associates, 1994, pp 15–37Google Scholar
33. Katz J: Classification of auditory processing disorders, in Central Auditory Processing: A Transdisciplinary View. Edited by Katz J, Stecker N, Henderson H. St Louis, Mosby, 1992, pp 81–91Google Scholar



