Association of Aggressive Behavior With Altered Serotonergic Function in Patients Who Are Not Suicidal
Abstract
OBJECTIVE: The purpose of this study was to determine whether aggression and serotonergic dysfunction are related in the absence of a history of suicidal behavior. Although serotonergic dysfunction has been implicated in aggressive and impulsive behavior, most studies of such behavior have included individuals with a history of suicide attempts. Low concentrations of CSF 5-hydroxyindoleacetic acid (5-HIAA) have been consistently associated with suicidal behavior, presenting a potential confound in the link between aggression and serotonergic dysfunction. METHOD: The authors examined the association between aggression and CSF 5-HIAA concentrations in a group of 64 patients who had different DSM-III-R axis I diagnoses and no past suicidal behavior. Aggressive (N=35) and nonaggressive (N=29) groups were defined by a median split on a six-item history of adulthood aggressive behavior. RESULTS: The aggressive group had significantly lower CSF 5-HIAA concentrations than the nonaggressive group. Aggressive individuals also scored significantly higher on self-report measures of hostility, impulsiveness, and sensation seeking. CSF 5-HIAA concentrations, however, did not correlate with self-reported hostility and impulsivity. CONCLUSIONS: There is an association between aggressive behavior and serotonergic dysfunction independent of suicidal behavior in patients with axis I disorders who exhibit relatively milder forms of aggressive behavior. Analogous to findings with suicidal behavior, a low concentration of CSF 5-HIAA is related to aggressive behavior but does not show the same relationship to the continuum of aggressive feelings and thoughts.
Aggressive behavior is a complex phenomenon, and both psychosocial and biological factors have been implicated as determinants (1–8). With respect to biology, a low concentration of the serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA) has been found in the CSF of violent and aggressive individuals (3–5, 7, 8). Brown et al. (3, 4) were the first to identify an inverse correlation between CSF 5-HIAA concentration and a lifetime history of aggression in individuals with personality disorders. A similar relationship between aggression and low CSF 5-HIAA concentrations was subsequently reported for impulsive murderers (7), arsonists (8), and individuals who committed infanticide (9). This relationship was further substantiated in a follow-up study in which recidivists were found to have lower levels of CSF 5-HIAA concentrations than nonrecidivists (10). Using prolactin response to fenfluramine as a measure of central serotonergic function, Coccaro et al. (11, 12) reported an association between measures of aggression and impulsiveness and a blunted prolactin response.
Taken together, these findings support the importance of altered central serotonergic function in impulsive, aggressive, and violent behavior. However, findings of more recent studies are at odds with earlier findings in reporting either higher levels of CSF 5-HIAA in aggressive individuals (13, 14) or the absence of a relationship between aggression and serotonergic functioning (12, 15). Thus, the role of serotonin in aggressive behavior remains unclear. Furthermore, although CSF 5-HIAA may be a factor in aggression, the importance of other biological and psychosocial variables must be considered.
Central serotonergic dysfunction has been linked consistently to suicidal behavior. More than 20 studies have reported low concentrations of CSF 5-HIAA in suicide attempters with a range of diagnoses, making this one of the strongest findings in biological psychiatry (11, 16). Suicide attempters have also been found to have a blunted prolactin response to fenfluramine (11). Consistent with antemortem findings, many postmortem studies have shown reduced density of cortical serotonin transporter sites and greater density of cortical postsynaptic serotonin 5-HT1A and 5-HT2A receptors in suicide victims (17–20). Thus, altered central serotonergic function is clearly associated with suicidal behavior.
Given the frequency of suicidal behavior in individuals with a history of aggression, the relationship between suicidality and central serotonergic dysfunction may obscure the association between serotonin and aggression. Therefore, an examination of the relationship between aggression and serotonergic function should require exclusion of individuals with a history of suicidal behavior. The importance of this strategy is underscored by the findings of Brown et al. (3, 4), who noted that CSF 5-HIAA concentrations were lowest in those individuals with both suicidal and aggressive behavior. Studies examining the relationship between impulsive aggressive behavior and CSF 5-HIAA have included samples in which 30%–80% of the subjects had a history of suicidal behavior (7, 8, 15). Other reports (12, 21) did not indicate the presence or absence of past suicidal behavior. Therefore, results of such studies regarding the relationship between CSF 5-HIAA and aggression may be explained, in part, by the inclusion of suicidal patients.
With respect to diagnosis, most previous studies of aggression and serotonergic dysfunction have examined a single diagnostic group, usually subjects with personality disorders (3–5, 12, 14, 15). Furthermore, these studies included individuals with more severe disorders who displayed frequent and serious forms of aggression such as arson and homicide, thus limiting the generalizability of their findings. Aggressive behavior is present in many diagnostic groups, including subjects with schizophrenia and mood disorders (22, 23); consequently, any biochemical abnormality associated with aggression is more meaningful if it is independent of diagnosis. Therefore, in the present study, we excluded subjects with a history of suicidal behavior and included a cross-section of patients with axis I diagnoses who exhibited less serious forms of aggressive behavior than usually studied, such as arguments with work supervisors, occasional physical fights, or property assaults.
In the present study, we hypothesized that 1) aggressive behavior is associated with reduced central serotonergic function in individuals who do not have a history of suicidal behavior, 2) this association exists in individuals with milder and less frequent aggressive behavior, and 3) the relationship between aggressive behavior and serotonergic dysfunction is present in a diverse diagnostic group.
METHOD
Subjects
Sixty-four psychiatric patients participated in the study after giving their written informed consent. The study was approved by the institutional review board at our facility. All patients underwent a physical examination and standard laboratory tests to rule out contraindications to participation. Exclusion criteria included a history of at least one suicide attempt; a diagnosis of current alcohol or substance abuse; a cognitive disorder that interfered with the patient’s ability to effectively answer clinician-administered and self-report rating scales; a history of head trauma resulting in coma; mental retardation or other significant cognitive impairment; and physical conditions precluding a lumbar puncture. Patients who had been receiving medications affecting the central nervous system entered a drug-free period of at least 14 days before the lumbar puncture was performed.
All patients had been admitted to a psychiatric inpatient unit and had a DSM-III-R diagnosis of 1) schizophrenia or schizoaffective disorder, 2) major depressive or dysthymic disorder, or 3) bipolar disorder.
Diagnostic and Behavioral Assessment
Patients were assigned DSM-III-R diagnoses following interviews, with a trained clinician, that included the Schedule for Affective Disorders and Schizophrenia (SADS) (24), the Schedule for Interviewing Borderlines (25), and a clinical interview to obtain the information required to convert Research Diagnostic Criteria diagnoses to DSM-III-R diagnoses. Raters were trained to an acceptable level of reliability by using videotaped assessments. Interrater reliability for the primary diagnosis was high (kappa=0.90).
Aggression was assessed by using a six-item semistructured interview, modified from the scale of Brown et al. (3), evaluating the history of aggressive behavior in adulthood. Childhood and adolescent aggressive behaviors were excluded. The behaviors included 1) physical fights, 2) assaults on people, 3) assaults against property, 4) difficulty in getting along with supervisors at work, 5) antisocial behavior involving the police, and 6) antisocial behavior that did not involve the police. Each item was rated on a frequency scale of 0 to 3 on which 0=the behavior never occurred, 1=the behavior occurred once, 2=the behavior occurred two to three times, and 3=the behavior occurred four or more times. The possible total score on the scale ranged from 0 to 18. Aggression history raters were blind to diagnosis, and interrater reliability was acceptable (r=0.82).
The Buss-Durkee Hostility Inventory (26) served as a measure of self-assessment and included eight subscales: assault, irritability, resentment, indirect hostility, negativism, suspiciousness, verbal hostility, and guilt. The history of a past suicide attempt was ruled out by reviewing the material from the SADS, the Schedule for Interviewing Borderlines, a clinical interview, and a semistructured interview that specifically questioned each individual regarding a history of a previous suicide attempt.
Impulsiveness was assessed by the nine-item impulsivity subscale of the Schedule for Interviewing Borderlines, which primarily measures nonaggressive impulsive behavior such as overspending, gambling, and impulsive sexual behavior (25), and by the Zuckerman Sensation Seeking Inventory (27), which includes four subscales: thrill and adventure seeking, experience seeking, disinhibition, and boredom susceptibility.
Additional measures of psychopathology included the Brief Psychiatric Rating Scale (BPRS) (28), which assesses psychotic symptoms, the Hamilton Depression Rating Scale (29), and the Hamilton Anxiety Rating Scale (30).
Lumbar Puncture and Determination of CSF 5–HIAA Level
The lumbar puncture was performed between 8:00 a.m. and 10:00 a.m., following a night of supervised bed rest and a fast of at least 8 hours. Before the lumbar puncture, patients were in bed in a prone position. The lumbar puncture was carried out under sterile conditions by a research psychiatrist using a fine-gauge (20 G) spinal needle, with the patient lying in a left lateral knee-chest position. CSF samples were consistently obtained from the space between the third and fourth lumbar vertebrae because 5-HIAA levels vary according to the site of lumbar punctures (31). Eighteen ml of CSF were collected in three aliquots of 2 ml, 15 ml, and 1 ml. The 15-ml aliquot was centrifuged (750 g for 5 minutes) and divided into 1-ml aliquots. All aliquots were stored at –80°C until assayed.
CSF 5-HIAA levels were determined in one of the 1-ml aliquots from the 15-ml sample by using high performance liquid chromatography with electrochemical detection (32). The coefficient of variance for 5-HIAA was 2.8% for the intraassay variability and 6.0% for the interassay variability.
Data Analysis
Patients were divided into aggressive and nonaggressive groups by a median split on aggression history scores. The aggressive group consisted of individuals with an aggression score equal to or greater than the median; the nonaggressive group had scores below the median.
The groups were compared on demographic, behavioral, psychopathological, and biological measures by using two-tailed Student’s t tests or chi-square analysis, where appropriate. Group differences were tested at the p<0.05 level. In an effort to attenuate the overall type I error rate, we did not compare groups on subscale scores unless total scale scores were significantly different. Mean CSF 5-HIAA levels were compared by using a two-tailed Student’s t test; an analysis of covariance (ANCOVA) with gender and height as covariates was also performed. The effect of diagnosis on CSF 5-HIAA levels and on aggression history scores was assessed by using a one-way analysis of variance.
RESULTS
The mean lifetime aggression history score of the total group of patients (N=64) was 2.9 (SD=2.5, median=3.0). Using a median split, we defined aggressive patients as those with a score of 3.0 or higher and the nonaggressive patients as those with scores less than 3.0. Given that the possible range of scores was 0–18, the aggressive group, with a mean score of 4.7 (SD=1.8), clearly committed aggressive acts that were relatively mild and infrequent.
The demographic and diagnostic characteristics of the study participants are summarized in table 1. The aggressive and nonaggressive patients did not differ in age, height, race, educational level, number of psychiatric hospitalizations, or diagnostic category, but the aggressive group had a higher proportion of men (66%) than the nonaggressive group.
When mean CSF 5-HIAA levels of the two groups were compared, the aggressive group was found to have significantly lower CSF 5-HIAA concentrations (93.5 pmol/ml, SD=36.2) than the nonaggressive group (116.6 pmol/ml, SD=40.3) (t=2.07, df=62, p<0.05). Means for men and women in each group are presented in figure 1. To determine whether the association between levels of CSF 5-HIAA and aggression in the present study group could be attributed to sex and/or height, we performed an ANCOVA using sex and height as covariates. The difference in CSF 5-HIAA levels remained significant (for the aggressive patients, adjusted mean=95.0 pmol/ml, SD=37.9; for the nonaggressive patients, adjusted mean=114.7 pmol/ml, SD=38.1) (F=3.90, df=2, 57, p=0.05). With respect to diagnosis, neither CSF 5-HIAA levels (F=2.24, df=2, 59, n.s.) nor aggression history scores (F=1.13, df=2, 59, n.s.) differed across diagnostic categories.
Self-report measures of behavioral and emotional aggressiveness, hostility, impulsivity, and overall psychopathology are summarized in table 2. The aggressive group had significantly higher overall levels of hostility as measured by the Buss-Durkee inventory. When we examined the subscales of this measure, the aggressive group scored significantly higher on assaultiveness, indirect hostility, verbal hostility, and negativism subscales. The aggressive group also had nonsignificantly higher scores on the irritability and resentment subscales. In examining other measures, we found that the aggressive individuals scored significantly higher on the Zuckerman Sensation Seeking Inventory, which could be attributed to the subscales of thrill/adventure seeking, disinhibition, and boredom susceptibility. The aggressive patients also obtained higher scores on the impulsiveness subscale of the Schedule for Interviewing Borderlines.
With regard to other forms of psychopathology (table 2) there were no significant group differences in Hamilton depression and anxiety scale scores, but the aggressive patients scored higher on the BPRS. When the BPRS subscales were analyzed, only the hostility/suspiciousness scores differed between groups. This scale consists of three items: hostility, uncooperativeness, and suspiciousness. This finding is consistent with the higher scores of the aggressive group on the Buss-Durkee subscales of hostility and negativism. The difference in total BPRS scores between the two groups seems to reflect group differences in the degree of hostility rather than psychotic symptoms. Thus, although the groups differed on aggression-related measures, they did not differ in overall psychopathology measures unrelated to aggression. Interestingly, although CSF 5-HIAA levels were lower in the aggressive group, these levels did not correlate with hostility as measured by the Buss-Durkee inventory (r=0.06, df=59, n.s.), the Zuckerman Sensation Seeking Inventory (r=0.07, df=60, n.s.), or the Schedule for Interviewing Borderlines impulsivity subscale (r=–0.02, df=44, n.s.). This reinforces the idea that aggressive acts, rather than aggressive thoughts and feelings per se, are related to altered serotonergic functioning.
DISCUSSION
The results of the present study indicate that aggressive behavior exhibited by patients with axis I disorders who have no history of suicide attempts is related to altered serotonergic function. We found that aggressive behavior was associated with lower concentrations of CSF 5-HIAA in a diagnostically heterogeneous patient population. Further, patients with higher levels of aggressive behavior and lower concentrations of CSF 5-HIAA were found to be more impulsive than their counterparts with lower levels of aggression.
The present study extends previous research that examined aggression and CSF 5-HIAA concentrations by controlling for the effect of suicidal behavior and by studying patients who did not exhibit more severe forms of aggressive behavior such as homicide and arson. The finding of an association between aggressive behavior and low CSF 5-HIAA levels is consistent with the finding of several previous reports of a similar relationship in individuals with personality disorders (3, 4), impulsive violent offenses (5), and a history of arson (8). In earlier studies, aggressive patients with personality disorders who had low CSF 5-HIAA also had a history of suicidal behavior (3, 4). Furthermore, the majority of impulsive violent offenders (5) and arsonists (8) also had a history of suicide attempts. Thus, it has been difficult to determine the extent to which alterations in serotonergic function are related to suicidal behavior, aggression, or both. On the basis of our findings, it appears that aggression may be related to serotonergic dysfunction independent of a history of suicidal behavior, which is consistent with a previous report of arsonists who had never attempted suicide and showed significantly lower concentrations of CSF 5-HIAA than a control group (8).
Our findings are at odds with those of Coccaro et al. (12), who reported a lack of association between CSF 5-HIAA and aggressive behavior. There are several possible reasons for these discrepant findings. First, our study excluded a history of suicide attempts, whereas Coccaro et al. (12) did not. Second, we are studying somewhat different patients. Coccaro et al. (12) studied patients with personality disorders and excluded the more severe axis I disorders. Our study group included patients with serious axis I disorders such as schizophrenia and bipolar disorder. It is possible that the presence of an axis I disorder potentiates the relationship between serotonergic dysfunction and lesser forms of aggressive behavior. Limson et al. (33) supported this notion by suggesting that the strength of the correlation between CSF 5-HIAA level and behavior weakens as we move from pathological to more normal subjects. Alternatively, when aggressive behavior is an aspect of an enduring personality pattern, other causative factors may be important.
In the present study, the aggressive group showed more impulsiveness than the nonaggressive diagnostic comparison subjects. This finding is consistent with previous reports of an association between central serotonergic dysfunction and impulsiveness and aggression (4, 5, 8, 10). The fact that aggressive behavior is related to serotonergic dysfunction, independent of suicidal behavior, plus the fact that aggression and suicide are associated with each other, suggests that the two behaviors may also have a common behavioral denominator, such as impulsiveness, which is associated with a common biological correlate (34).
Nonhuman primate studies have also found a relationship between low CSF 5-HIAA concentrations and impulsive aggression. A propensity for aggression has been found in domestic dogs who have lower serotonergic activity (35). Since animals may be presumed to be free from major psychiatric disorders, animal studies demonstrate the generalizability of the relationship between lower serotonin activity and impulsive aggression and support our conclusion that the relationship is independent of suicidal behavior.
Aggression, like suicidal behavior, is not restricted to a single diagnostic category (22), and the present study examined this behavior in a diagnostically heterogeneous group of psychiatric patients. By doing so, we have shown that the association between aggression and low CSF 5-HIAA concentrations is not restricted to personality disorders alone but can be generalized to include a spectrum of diagnoses, such as depression, bipolar disorder, and schizophrenic disorders.
In conclusion, the present study shows that aggression is associated with reduced central serotonergic function independent of suicidal behavior. Our findings suggest that impulsive aggression may be treatable by pharmacological manipulation of the serotonergic system. However, the relationship between serotonergic dysfunction and aggression does not diminish the importance of psychological and environmental factors as additional determinants of aggressivity. Furthermore, other neurotransmitters that may be important remain to be studied.
Received Feb. 26, 1999; revision received Aug. 18, 1999; accepted Aug. 25, 1999. From the Department of Psychiatry, College of Physicians and Surgeons, Columbia University, New York; and the Department of Neuroscience, New York State Psychiatric Institute. Address reprint requests to Dr. Barbara Stanley, Unit 42, New York State Psychiatric Institute, 1051 Riverside Dr., New York, NY 10032. Dr. Michael Stanley is deceased. Supported in part by NIMH Public Health Service grant MH-41847 (Dr. Barbara Stanley) and MH-46745 (Dr. Mann).
 |
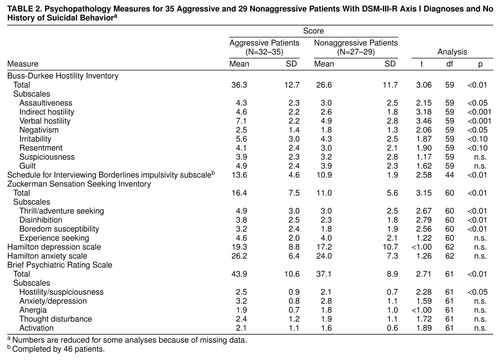 |
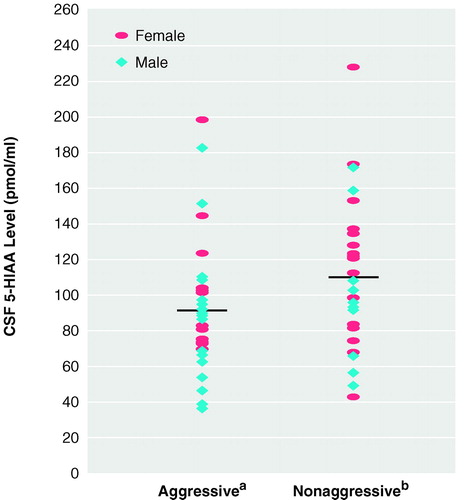
FIGURE 1. CSF 5-HIAA Levels of 35 Aggressive and 29 Nonaggressive Patients With DSM-III-R Axis I Diagnoses and No History of Suicidal Behavior
aFor men, mean=88.0 pmol/ml (SD=34.1), and for women, mean=103.9 pmol/ml (SD=10.9) (t=–1.26, df=33, n.s.). Horizontal line represents group mean.
bFor men, mean=102.3 pmol/ml (SD=36.2), and for women, mean=121.0 pmol/ml (SD=43.4) (t=–1.23, df=27, n.s.). Horizontal line represents group mean.
1. Geen RG, Donnerstein EL (eds): Aggression: Theoretical and Methodological Issues. New York, Academic Press, 1983Google Scholar
2. Goldstein JH: Aggression and Crimes of Violence, 2nd ed. New York, Oxford University Press, 1986Google Scholar
3. Brown GL, Goodwin FK, Ballenger JC, Goyer PF, Major LF: Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Res 1979; 1:131–139Crossref, Medline, Google Scholar
4. Brown GL, Ebert MH, Goyer PF, Jimerson DC, Klein WJ, Bunney WE, Goodwin FK: Aggression, suicide, and serotonin: relationships to CSF amine metabolites. Am J Psychiatry 1982; 139:741–746Link, Google Scholar
5. Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK: Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci 1983; 33:2609–2614Google Scholar
6. Bioulac B, Benezech M, Renaud B, Noel B, Roche D: Serotoninergic dysfunction in the 47, XYY syndrome. Biol Psychiatry 1980; 15:917–923Medline, Google Scholar
7. Lidberg L, Tuck JR, Asberg M, Scalia-Tomba GP, Bertilsson L: Homicide, suicide and CSF 5-HIAA. Acta Psychiatr Scand 1985; 71:230–236Crossref, Medline, Google Scholar
8. Virkkunen M, Nuutila A, Goodwin FK, Linnoila M: Cerebrospinal fluid monoamine metabolite levels in male arsonists. Arch Gen Psychiatry 1987; 44:241–247; correction, 1989; 46:960Google Scholar
9. Lidberg L, Asberg M, Sundqvist-Stensman UB:5-Hydroxyindoleacetic acid levels in attempted suicides who have killed their children (letter). Lancet 1984; 2:928Google Scholar
10. Virkkunen M, De Jong J, Bartko JJ, Goodwin FK, Linnoila M: Relationship of psychobiological variables to recidivism in violent offenders and impulsive fire setters: a follow-up study. Arch Gen Psychiatry 1989; 46:600–603; correction, 46:913Crossref, Medline, Google Scholar
11. Coccaro EF, Siever LJ, Klar HM, Maurer G, Cochrane K, Cooper TB, Mohs RC, Davis KL: Serotonergic studies in patients with affective and personality disorders: correlates with suicidal and impulsive aggressive behavior. Arch Gen Psychiatry 1989; 46:587–599; correction, 1990; 47:124Google Scholar
12. Coccaro EF, Kavoussi RJ, Cooper TB, Hauger RL: Central serotonin activity and aggression: inverse relationship with prolactin response to d-fenfluramine, but not CSF 5-HIAA concentration, in human subjects. Am J Psychiatry 1997; 154:1430–1435Google Scholar
13. Moller SE, Mortensen EL, Breum L, Alling C, Larsen OG, Boge-Rasmussen T, Jensen C, Bennicke K: Aggression and personality: association with amino acids and monoamine metabolites. Psychol Med 1996; 26:323–331Crossref, Medline, Google Scholar
14. Coccaro EF: Impulsive aggression and central serotonergic system function in humans: an example of a dimensional brain-behavior relationship. Int Clin Psychopharmacol 1992; 7:3–12Crossref, Medline, Google Scholar
15. Coccaro EF, Kavoussi RJ, Hauger RL, Cooper TB, Ferris CF: Cerebrospinal fluid vasopressin levels: correlates with aggression and serotonin function in personality-disordered subjects. Arch Gen Psychiatry 1998; 55:708–714Crossref, Medline, Google Scholar
16. Molcho A, Stanley B, Stanley M: Biological studies and markers in suicide and attempted suicide. Int Clin Psychopharmacol 1991; 6:77–92Crossref, Medline, Google Scholar
17. Stanley M, Virgilio J, Gershon S: Tritiated imipramine binding sites are decreased in the frontal cortex of suicides. Science 1982; 216:1337–1339Google Scholar
18. Stanley M, Mann JJ: Increased serotonin-2 binding sites in frontal cortex of suicide victims. Lancet 1983; 1:214–216Crossref, Medline, Google Scholar
19. Arango V, Underwood MD, Gubbi AV, Mann JJ: Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res 1995; 688:121–133Crossref, Medline, Google Scholar
20. Arango V, Underwood MD, Mann JJ: Postmortem findings in suicide victims: implications for in vivo imaging studies, in The Neurobiology of Suicide: From the Bench to the Clinic. Edited by Stoff DM, Mann JJ. New York, New York Academy of Sciences, 1997, pp 269–287Google Scholar
21. Stanislav SW, Crismon ML, Childs NL: Cerebrospinal fluid monoamine metabolites and glucose metabolism in posttraumatic aggression. Biol Psychiatry 1998; 43:619–621Crossref, Medline, Google Scholar
22. Krakowski M, Volavka J, Brizer D: Psychopathology and violence: a review of the literature. Compr Psychiatry 1986; 27:131–148Crossref, Medline, Google Scholar
23. Asberg M, Thoren P, Traskman L, Bertilsson L, Ringberger V: “Serotonin depression”—a biochemical subgroup within the affective disorders? Science 1976; 191:478–480Google Scholar
24. Spitzer RL, Endicott J: Schedule for Affective Disorders and Schizophrenia (SADS), 3rd ed. New York, New York State Psychiatric Institute, Biometrics Research, 1978Google Scholar
25. Baron M: The Schedule for Interviewing Borderlines (SIB). New York, New York State Psychiatric Institute, 1980Google Scholar
26. Buss AH, Durkee A: An inventory for assessing different kinds of hostility. J Consult Psychol 1957; 21:343–349Crossref, Medline, Google Scholar
27. Zuckerman M: Sensation Seeking: Beyond the Optimal Level of Arousal. Hillsdale, NJ, Lawrence Erlbaum Associates, 1979Google Scholar
28. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 158–169Google Scholar
29. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 180–192Google Scholar
30. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 194–198Google Scholar
31. Nordin C, Siwers B, Bertilsson L: Site of lumbar puncture influences level of monoamine metabolites (letter). Arch Gen Psychiatry 1982; 39:1445Crossref, Medline, Google Scholar
32. Stanley M, Traskman-Bendz L, Dorovini-Zis K: Correlations between aminergic metabolites simultaneously obtained from human CSF and brain. Life Sci 1985; 37:1279–1286Google Scholar
33. Limson R, Goldman D, Roy A, Lamparski D, Ravitz B, Adinoff B, Linnoila M: Personality and cerebrospinal fluid monoamine metabolites in alcoholics and controls. Arch Gen Psychiatry 1991; 48:437–441Crossref, Medline, Google Scholar
34. Mann JJ: The neurobiology of suicide. Nat Med 1998; 4:25–30Crossref, Medline, Google Scholar
35. Reisner IR, Mann JJ, Stanley M, Huang YY, Houpt KA: Comparison of cerebrospinal fluid monoamine metabolite levels in dominant-aggressive and nonaggressive dogs: Brain Res 1996; 714:57–64Google Scholar



