Chronic Late-Onset Schizophrenia-Like Psychosis That Remitted: Revisiting Newton’s Psychosis?
According to historical records, Sir Isaac Newton developed new-onset psychosis at age 51, which was characterized by persecutory delusions. His psychotic symptoms remitted in less than 18 months, but their etiology has since remained a puzzle. The present case conference involves a present-day individual with late-onset paranoid psychosis with later remission, which has some interesting similarities to, as well as important differences from, Newton’s illness. Our patient had no history of psychiatric illness until age 52, when he developed paranoid delusions, which included thought broadcasting, auditory hallucinations, blunted affect, and poor insight. His early medical history was remarkable for grand mal seizures between ages 15 and 29. His psychosis improved with the use of antipsychotic medication. Approximately 6 years after its onset, his psychotic illness remitted, and all psychotropic medications were discontinued. Four years later, at the writing of this report, our patient continued to function independently with no psychiatric symptoms. We discuss the differential diagnosis for this chronic late-onset schizophrenia-like psychosis with remission.
Historical records suggest that in 1693, Sir Isaac Newton, the renowned scientist, experienced an episode of psychosis at the age of 51 that was characterized by paranoid delusions, insomnia, irritability, and loss of appetite (1, 2). He wrote several letters in which he cut ties with close friends and colleagues and made various accusations and references to conversations that never occurred. For example, one letter was written to his friend and colleague Samuel Pepys on September 13, 1693.
Sir, Some time after Mr. Millington…had delivered your message, he pressed me to see you the next time I went to London. I was averse; but upon his pressing consented, before I considered what I did, for I am extremely troubled at the embroilment I am in, and have neither ate nor slept well this twelve month, nor have I my former consistency of mind.…I must withdraw from your acquaintance, and see you nor the rest of my friends any more. (quoted in Christianson [1, pp 355–356])
Pepys was innocent of the accusations Newton alluded to and was quite shaken by his letter. Moreover, the conversation with Millington that Newton refers to apparently never occurred (1).
Shortly thereafter, Newton wrote a letter to John Locke, a philosopher and Newton’s personal friend, in which Newton admitted that he had been of the opinion that Locke was attempting to “embroil him with women.” The news of Newton’s “mental breakdown” spread widely. One German philosopher wrote that he had heard that Newton was “so disturbed in mind…as to be reduced to very ill circumstances” (quoted in White [2, p. 252]).
The records of Newton’s illness are too spotty to give us certainty as to precisely what symptoms he experienced and when. Nonetheless, it appears that the psychosis lasted for less than 18 months. When his subsequent remission was complete, Newton returned to his productive scientific career. He expressed remorse to his friends for his accusations (2).
The cause of Newton’s psychosis has since remained a mystery. Although some authors have attributed Newton’s illness to metal poisoning secondary to his experiments in alchemy, Christianson (1) presents excellent evidence against this theory. For example, Newton appears to have manifested few of the common symptoms of poisoning, such as tremor or ulceration of the gums and loosening of the teeth. Moreover, Newton recovered within 18 months, when he was still devoting considerable time to his work in alchemy. Others have attributed his psychosis to the breakup of an intimate relationship with a young male scientist, loss of important papers in a fire, and/or other professional stresses and frustrations that were occurring around this time (1, 2). The trail of evidence is probably too cold for us to ever know definitively what this illness was or what caused it. We now present a description of a contemporary individual with late-onset psychosis with later remission that has some interesting parallels to, as well as differences from, Newton’s psychosis.
CASE PRESENTATION
Initial Symptoms
Mr. A had no history of psychiatric symptoms or legal problems until his early 50s, when he was incarcerated for a nonviolent offense for 6 months. During the incarceration, his mental status deteriorated. He began exhibiting tangential and disorganized thinking and paranoid delusions. He was hospitalized in the prison infirmary and treated with haloperidol, 10 mg b.i.d., nortriptyline, 100 mg/day, phenytoin, 300 mg/day (because of a past history of seizures), amantadine, 100 mg b.i.d., and metoprolol tartrate, 100 mg/day (for hypertension). Medical records from the correctional facility indicate that his symptoms responded well to treatment. He was released after serving his full sentence.
A day after his release, Mr. A experienced a relapse. He was agitated, wandered at night, stared at others, was noncommunicative, and had a poor appetite. He believed that other people could read and broadcast his thoughts. He also heard voices outside his window telling him that he was “no good” and would “never amount to anything.” Mr. A heard his neighbors talking about him and actually confronted some individuals, which resulted in an altercation. His family sought inpatient treatment for Mr. A.
Family and Developmental History
Mr. A was the oldest of five siblings. He had three sisters, a brother, and several half-siblings. Mr. A’s mother, a homemaker, died of pneumonia in her early 30s. His father, a blue-collar worker, was in his mid-80s at the time of this report and was functioning independently.
Mr. A reported no significant family problems or discord in his childhood. His father reportedly had a history of alcohol dependence, one uncle had a seizure disorder, and another uncle had possible dementia and depression.
Mr. A dropped out of school in the 10th grade at age 16. He then joined the Navy and served for 9 years as a cook and baker. He reported having several close friends, both men and women, and three long-term relationships. After an honorable discharge from the Navy, Mr. A joined the civil service and worked for about 20 years as a janitor, warehouse worker, truck driver, and food server. He was married in his late 20s. After about 15 years of marriage, he and his wife separated, but he reported that they were still friends. They had three children.
Premorbid Medical History
Mr. A’s early medical charts indicate that he had seizures at ages 15, 17, 19, and 29. He experienced an aura with headaches, blurred vision, dizziness, and a “swimming feeling” in his head 15–20 minutes before the onset of a seizure. During one seizure he fell to the ground and sustained injuries of an unspecified nature. Mr. A was reported to “shake,” remain unconscious for a few minutes, and then appear dazed for a few additional minutes. There was no tongue bite reflex or incontinence. Mr. A had no recollection of the nature of his seizures. The results of an EEG performed at age 30 revealed diffuse slowing and excessive paroxysmal activity during the hyperventilation and posthyperventilation phases of the recording. The EEG was reported as being compatible with, but not diagnostic of, a seizure disorder.
Mr. A. was diagnosed with granulomatous disease of the lungs at age 31. He had a history of light cigarette smoking and social drinking but stopped both at age 48, when he was found to have hypertension and hyperlipidemia.
Initial Examination
At the time of his first psychiatric assessment after incarceration, Mr. A was noted to be staring blankly without looking at the interviewer. His speech was monosyllabic, and his affect was blunted. He appeared to be responding to internal stimuli, although he denied experiencing auditory hallucinations. He was oriented to time, place, and person but had poor insight and judgment. Results of a neurological examination revealed a decreased upward gaze, positive glabella tap, decreased deep tendon reflexes in both lower extremities, and a resting tremor. He had decreased sensation to pin prick in a glove-and-stocking pattern and had ataxia on tandem gait. A noncontrast computerized tomography scan showed no structural brain lesions.
Treatment and Course of Illness
Mr. A was hospitalized and began treatment with haloperidol, 5 mg b.i.d., and nortriptyline, 100 mg at bedtime. Because of a history of seizures, he was also given—as a precautionary measure—phenytoin, 300 mg/day. Three days later, his treatment with phenytoin and nortriptyline was discontinued. Mr. A’s mental status improved progressively, and he became cooperative and well groomed. At that point, treatment with haloperidol was discontinued. Mr. A’s condition began to deteriorate. A trial of haloperidol plus desipramine was initiated, in addition to treatment with triamterene and gemfibrozil for hypertension and hyperlipidemia, respectively.
After 6 weeks of treatment, Mr. A’s condition stabilized, and he was discharged to live with his daughter. His haloperidol treatment was discontinued, presumably because of the remission of his psychotic features. Mr. A continued outpatient treatment. He did reasonably well for a year, at which point he began to decompensate and experienced auditory hallucinations and multiple conversant voices everyday for much of the day. Mr. A also noted feelings of electricity or rays going through his body. He complained of thought insertion. He was readmitted to the inpatient psychiatric unit for 2 weeks. The psychiatrist there (not a member of our team) made a diagnosis of chronic paranoid schizophrenia. A mental status examination revealed a rumpled appearance, blank stare, decreased movements, and paucity of thought content. Mr. A denied feeling depressed, and his affect appeared blunted. He was given haloperidol decanoate, 50 mg i.m. He became less withdrawn, and his hallucinations improved. When Mr. A was discharged, he was receiving haloperidol decanoate, 50 mg i.m. every 2 weeks, and enalapril for hypertension.
Over the following 8 months, Mr. A’s symptoms continued to improve. He reported a complete absence of auditory hallucinations and paranoia. His dose of haloperidol decanoate was reduced to 50 mg i.m. every 4 weeks. Five months later, Mr. A again experienced mild paranoia and increased insomnia. He was also noted to have akathisia and mild tardive dyskinesia. His dose of haloperidol decanoate was increased to 75 mg i.m. every 4 weeks, and treatment with trihexyphenidyl was initiated. Mr. A’s paranoia disappeared, although he continued to experience restlessness and tardive dyskinesia. A trial of vitamin E had little effect on his tardive dyskinesia.
After 18 months of treatment with haloperidol decanoate, Mr. A’s antipsychotic medication was changed to risperidone, 3 mg b.i.d. He remained on risperidone treatment for 2 years, although his dose was gradually reduced as his psychotic symptoms improved. He became symptom free at age 58. His psychotropic medications were discontinued several months later. Mr. A continued to live with his daughter and grandchildren until age 61, when he moved into an independent-living complex for seniors. His condition was followed up by our geriatric psychiatry clinic and research program. His most recent medications included fosinopril and hydrochlorothiazide/triamterene for hypertension and simvastatin for hyperlipidemia.
At the time of this report, approximately 4 years after Mr. A discontinued his psychotropic medications, he lived independently and helped drive his daughter to work and his grandchildren to school. He was responsible for all aspects of daily living, including paying bills, cooking, cleaning, and grooming. He enjoyed watching sports on television with friends and visiting with his ex-wife. He complained only of occasional insomnia and still had mild tardive dyskinesia.
Longitudinal Research Assessments
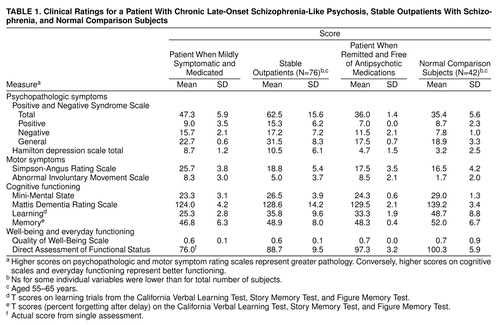
Mr. A was repeatedly assessed as a consenting outpatient at our clinic with standardized instruments for evaluating psychopathologic symptoms, movement abnormalities, cognitive impairment, well-being, and everyday functioning. Table 1 compares his mean scores over several assessments as a mildly symptomatic but medicated and clinically stabilized outpatient and as a medication-free patient in remission with the mean scores of similarly aged (55–65 years) outpatients with schizophrenia (N=76) and normal comparison subjects (N=42).
The overall severity of Mr. A’s psychopathologic symptoms on the Positive and Negative Syndrome Scale (3) and the Hamilton Rating Scale for Depression (4) as a medicated outpatient was relatively mild (i.e., his symptoms had already improved relative to their initial manifestation by our baseline assessment). (His records suggested that his symptoms had been much more severe during his hospitalization.) As Mr. A’s symptoms went into remission, his symptoms’ severity decreased further and became comparable to that of normal comparison subjects.
The severity of Mr. A’s extrapyramidal symptoms and dyskinesia was evaluated with the modified Simpson-Angus Rating Scale (5) and the Abnormal Involuntary Movement Scale (6), respectively. His scores on both scales were similar to those of other outpatients, and although the scores decreased somewhat after he discontinued his antipsychotic medication, he continued to have persistent extrapyramidal symptoms and dyskinesia.
Mr. A’s global cognitive performance was assessed with the Mini-Mental State examination (7) and the Mattis Dementia Rating Scale (8). His cognitive scores improved as his psychosis remitted, partly owing to practice effect, yet they were still much closer to those of the symptomatic outpatients than to those of the normal comparison subjects.
As a participant in a series of comprehensive neuropsychological evaluations (see 9, 10 for details), Mr. A also completed the California Verbal Learning Test (11) and the Story and Figure Memory Tests (9). Mr. A’s cognitive performance remained relatively stable throughout the follow-up period. Although his learning scores were in the mildly to moderately impaired range, he had consistently normal retention of learned material. This pattern is similar to that reported in patients with schizophrenia, but it is inconsistent with what has been reported in patients with Alzheimer’s disease (10).
Mr. A was also evaluated on the Quality of Well-Being Scale (12), a utility measure designed to assess health-related aspects of the quality of life, and the Direct Assessment of Functional Status (13), an observation-based measure of everyday functioning. Mr. A’s scores on the Quality of Well-Being Scale and the Direct Assessment of Functional Status as a symptomatic outpatient were comparable to those of other outpatients, whereas his more recent scores were similar to those of normal comparison subjects, which was consistent with observations of clinical remission.
A recent magnetic resonance image showed mild central and peripheral volume loss consistent with Mr. A’s age and minimal scattered periventricular deep white matter hyperechoic foci consistent with chronic ischemic change. The results of a recent sleep EEG with nasopharyngeal leads revealed mild nonspecific abnormalities that were reported to have minimal clinical significance.
DISCUSSION
Our patient had no previous history of psychiatric illness until age 52. He was seen with auditory hallucinations, thought broadcasting, and other paranoid delusions. His medical history was remarkable for a seizure disorder. His neuropsychological performance did not suggest dementia. He was treated with an antipsychotic, an antidepressant, and an anticonvulsant but ultimately seemed to benefit most from antipsychotic medication. He developed the symptoms of tardive dyskinesia within a year of treatment with haloperidol decanoate. Approximately 6 years after the onset of his psychiatric symptoms, our patient experienced remission of his psychiatric symptoms, and his psychotropic medications were discontinued. Nearly 4 years later, at the time of this report, he continued to function independently, with no psychiatric symptoms, but he still had some persistent extrapyramidal symptoms and tardive dyskinesia.
Diagnostic Implications
Our patient’s differential diagnosis included the following:
1. Psychotic disorder due to a general medical condition (seizure disorder). Our patient’s history and the results of a physical examination and laboratory assessments provided no evidence of any medical condition that likely caused his psychotic illness other than seizure disorder.
An increased incidence of psychotic symptoms in patients with seizure disorders has been observed for more than a century (14). Mesial temporal lobes are often implicated in the psychopathology of schizophrenia and are also a frequent point of origin for complex partial seizures (15). There is a higher risk of schizophrenia in patients with complex partial seizures than in those with generalized tonic-clonic seizures (16). Psychosis may follow the onset of epilepsy, during an interval ranging from 14 to 21 years (15). Some researchers have hypothesized that complex partial seizures may induce a neurologic reorganization that subsequently predisposes patients to psychosis (17).
Our patient’s seizures were, however, of the grand mal type, and his last known seizures occurred 23 years before the onset of his psychotic symptoms. While he may have continued to experience subtle seizures, the results of a recent EEG did not suggest an ongoing seizure disorder. Finally, antipsychotics, rather than anticonvulsants, proved to be the best treatment for his psychotic symptoms. Our patient’s seizure disorder (or its underlying neuropathological cause) might have made him vulnerable to subsequent psychosis, but a diagnosis of psychosis secondary to a seizure disorder would be difficult to justify in this case.
2. Dementia of the Alzheimer’s type with delusions and hallucinations. Psychotic symptoms that first appear in late life may be symptoms of Alzheimer’s disease or other dementing disorders (18). Our patient’s neuropsychological performance was, however, not consistent with that of Alzheimer’s disease. One of the hallmarks of Alzheimer’s disease is rapid forgetting, but our patient showed consistently normal retention of material, once learned, on measures of memory. In fact, his neuropsychological performance and his everyday functioning improved when his status was assessed over several years.
3. Brief reactive psychosis. Although our patient’s incarceration constituted a significant stressor in his life and was concurrent with the onset of his psychotic symptoms, he continued to experience symptoms years after his release. The persistence and duration of his psychotic symptoms precluded this diagnosis.
4. Factitious disorder or “hysterical psychosis” (19). It is possible that the onset of our patient’s psychosis was related to a conscious or unconscious motivation to gain early release from incarceration. Such motivation would suggest a diagnosis of factitious disorder, conversion reaction, or dissociative disorder. Yet our patient demonstrated improvement with treatment while incarcerated. In addition, he served his entire sentence and, in fact, experienced a severe psychotic episode and decline in function a year after his release. While the patient might have had a primary or secondary gain from the development of psychosis while incarcerated, there were no obvious environmental reasons for chronicity or eventual remission.
5. Mood disorder with psychotic features. Our patient never met the DSM-IV criteria for a manic or hypomanic episode or for major depressive episode. His affect was typically described as blunted or flat. There was no history of marked mood fluctuations.
6. Delusional disorder. Bizarre delusions, including thought insertion and prominent auditory hallucinations, ruled out this condition.
7. Schizophrenia. Onset of our patient’s psychotic symptoms at age 52 would have excluded this diagnosis in DSM-III, which explicitly prohibited the diagnosis of schizophrenia in cases with onset of prodromal symptoms after age 45. DSM-III-R permitted a diagnosis of late-onset schizophrenia in such cases. The DSM-IV criteria have no restrictions or specifiers based on age at onset, although the construct of late-onset schizophrenia has remained somewhat controversial (20–24).
Clinically, our patient seemed to meet the DSM-IV criteria for schizophrenia. As a medicated and mildly symptomatic outpatient, his ratings for psychopathologic symptoms, well-being, and everyday functioning were similar to those of other clinically stable outpatients with schizophrenia. As his symptoms remitted, however, our patient’s scores on these scales became similar to those of normal comparison subjects. While chronicity is the rule in schizophrenia, several investigators have found that as many as 20% of such patients experience remission in late life (25, 26). Certain factors have reportedly been associated with a positive long-term outcome in schizophrenia (27, 28). Our patient had several of these characteristics, including good premorbid adjustment, acute onset of psychosis, and early intervention with antipsychotic medications. His neuropsychological performance was similar to that seen in patients with schizophrenia rather than to that of patients with Alzheimer’s disease.
Our patient’s psychosis differed from that found in typical schizophrenia in several important ways. He had no family history of schizophrenia and had apparently normal premorbid functioning. A major stressor precipitated his first psychotic episode, and after discharge from the hospital, he did well in the absence of neuroleptic treatment for a year. His subsequent remission resulted in a return to completely normal premorbid functioning, without any of the “stigmata” of chronic schizophrenia. The confluence of all of these unusual features suggests that a diagnosis of late-onset schizophrenia-like psychosis might be appropriately less specific than that of schizophrenia.
8. Psychotic disorder not otherwise specified. Our patient could be given a diagnosis of psychotic disorder not otherwise specified since he did not fit the typical pattern of any common psychotic disorder. This diagnosis is one of exclusion, however, and does not help explain the nature of his illness.
Comparisons Between the Psychotic Illnesses of Newton and Our Patient
Any comparisons of the psychotic illnesses of Newton and our patient are severely limited because we have very poor documentation of the onset, nature, and course of Newton’s symptoms. Nonetheless, at least superficially, there are several interesting parallels between our patient’s psychosis and that reportedly exhibited by Newton. One is the lack of previous symptoms until a psychotic breakdown in their early 50s. A second similarity is the predominance of persecutory delusions. Newton believed that his friends and colleagues were trying to smear his reputation or were otherwise conspiring to hurt his position, and he made reference to conversations that never occurred. He angrily confronted his colleagues about this through his letters. Our patient believed that his neighbors were talking about him, and he actually confronted some individuals, which resulted in an altercation.
Another similarity is the murky nature of the etiology of the psychosis. In both cases there were suggestions of possible organic contributions (metal poisoning for Newton and seizure disorder for our patient), yet on close inspection, the organic explanations seemed unable to fully account for the psychosis. Thus, certain features that would have strongly supported a diagnosis of psychosis secondary to a general medical condition were absent (e.g., Newton did not exhibit the common signs of metal poisoning, and our patient’s history and EEG results did not suggest complex partial seizures). In both cases there were significant psychosocial stressors that might have precipitated the initial psychosis (Newton had experienced a breakup in a close personal relationship and also had professional problems, and our patient was incarcerated).
Finally, both men experienced total remission of their symptoms after months or years of psychotic illness and returned to their premorbid levels of functioning, with little evidence of residual symptoms.
There are, however, some important differences between these two cases. Our patient’s symptoms included auditory hallucinations, bizarre delusions (thought insertion and thought broadcasting), and negative symptoms, including blunted affect and paucity of thought content. Since the records of Newton’s symptoms consist primarily of letters to and among his acquaintances, it is difficult to know whether he experienced hallucinations and other delusions. Furthermore, while the precise onset of Newton’s psychosis is not clear, it appears that his symptoms spontaneously remitted in less than 18 months. In contrast, our patient did not experience full remission until 6 years after his initial episode, despite treatment with antipsychotics.
Relatively little research has been done on chronic late-onset psychotic disorders in patients without dementia or mood disorders. We believe that a proportion of such patients do have late-onset schizophrenia (22–24), whereas others have delusional disorder or other DSM-IV-defined primary psychotic disorders. There remain subgroups of patients, however, whose diagnostic categorization is uncertain. The use of terms such as “paraphrenia” or “psychotic disorder not otherwise specified” is unhelpful because these terms do not shed light on the nature of these psychotic disorders. The diagnosis of paraphrenia has been used so inconsistently over the years that it does not appear to have a clear meaning (29). When Kraepelin (30) used the term “paraphrenia,” his concept (which made no reference to age at onset) involved a form of psychosis in which patients manifested hallucinations and delusions, but other aspects of their mental functioning and personality were relatively spared. Roth (31) introduced the term “late paraphrenia” in the 1950s to refer to paranoid psychosis with onset after age 65. However, both terms (“paraphrenia” and “late paraphrenia”) were sometimes used as if they were synonymous with “late-onset schizophrenia.” Some prominent investigators in the United Kingdom, where this terminology has been historically more popular, have recently abandoned the term “paraphrenia” because of a lack of known distinct etiologic or treatment implications (Robert Howard, personal communication, August 1999).
Our patient would appear to meet the DSM-IV criteria for schizophrenia with late onset. In Newton’s case, the available information is inadequate to make a DSM-IV diagnosis. We would, however, not recommend a diagnosis of late-onset schizophrenia in either individual because of the temporally circumscribed nature of the psychosis, in which both men had relatively normal premorbid and postmorbid functioning. (Patients with late-onset schizophrenia tend to have somewhat subnormal premorbid functioning and generally have a chronic course of illness without full remission.) For the lack of a better term, one may call the disorder “late-onset schizophrenia-like psychosis.” Patients with such a disorder expose the limitations of our present diagnostic systems and call for more research on psychotic disorders with late-life onset that do not fit nicely into the standard nosologic schemas (24).
Received Aug. 9, 1999; revision received Dec. 13, 1999; accepted Dec. 21, 1999. From the Department of Psychiatry, University of California, San Diego; and the VA San Diego Healthcare System, San Diego. Address reprint requests to Dr. Jeste, Division of Geriatric Psychiatry, University of California, San Diego, VA San Diego Healthcare System, 116A-1, 3350 La Jolla Village Dr., San Diego, CA 92161; [email protected] (e-mail). Supported by NIMH grants MH-43693, MH-49671, MH-45131, MH-42522, and MH-01452 and by the U.S. Department of Veterans Affairs.
 |
1. Christianson GE: In the Presence of the Creator: Isaac Newton and His Times. New York, Free Press, 1984Google Scholar
2. White M: Isaac Newton: The Last Sorcerer. Reading, Mass, Addison-Wesley, 1997Google Scholar
3. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
4. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
5. Simpson GM, Angus JWS: A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 1970; 212:11–19Crossref, Medline, Google Scholar
6. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 534–537Google Scholar
7. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
8. Mattis S: Dementia Rating Scale. Odessa, Fla, Psychological Assessment Resources, 1973Google Scholar
9. Heaton RK, Grant I, Matthews CG: Comprehensive Norms for Expanded Halstead-Reitan Battery: Demographic Corrections, Research Findings, and Clinical Applications. Odessa, Fla, Psychological Assessment Resources, 1991Google Scholar
10. Heaton RK, Paulsen JS, McAdams LA, Kuck J, Zisook S, Braff D, Harris MJ, Jeste DV: Neuropsychological deficits in schizophrenia: relationship to age, chronicity, and dementia. Arch Gen Psychiatry 1994; 51:469–476Crossref, Medline, Google Scholar
11. Delis DC, Kramer JH, Kaplan E, Ober BA: California Verbal Learning Test (CVLT) Manual. San Antonio, Tex, Psychological Corp (Harcourt), 1987Google Scholar
12. Patterson TL, Kaplan RM, Grant I, Semple SJ, Moscona S, Koch WL, Harris MJ, Jeste DV: Quality of well-being in late-life psychosis. Psychiatry Res 1996; 63:169–181Crossref, Medline, Google Scholar
13. Loewenstein DA, Amigo E, Duara R, Guterman A, Hurwitz D, Berkowitz N, Wilkie F, Weinberg G, Black B, Gittelman B: A new scale for the assessment of functional status in Alzheimer’s disease and related disorders. J Gerontol 1989; 4:114–121Crossref, Google Scholar
14. Gibbs FA: Ictal and non-ictal psychiatric disorders in temporal lobe epilepsy. J Nerv Ment Dis 1951; 113:522–528Medline, Google Scholar
15. Hyde TM, Weinberger DR: Seizures and schizophrenia. Schizophr Bull 1997; 23:611–622Crossref, Medline, Google Scholar
16. Mendez M, Grau R, Doss R, Taylor J: Schizophrenia in epilepsy: seizure and psychosis variables. Neurology 1993; 43:1073–1077Google Scholar
17. Stevens JR: Abnormal reinnervation as a basis for schizophrenia: a hypothesis. Arch Gen Psychiatry 1992; 49:238–243Crossref, Medline, Google Scholar
18. Wragg RE, Jeste DV: Overview of depression and psychosis in Alzheimer’s disease. Am J Psychiatry 1989; 146:577–587Link, Google Scholar
19. Hirsch SJ, Hollender MH: Hysterical psychosis: clarification of the concept. Am J Psychiatry 1969; 125:909–915Link, Google Scholar
20. Andreasen NC: I don’t believe in late onset schizophrenia, in Late-Onset Schizophrenia. Edited by Howard R, Rabins PV, Castle DJ. Philadelphia, Wrightson Biomedical, 1999, pp 111–123Google Scholar
21. Crespo-Facorro B, Piven MLS, Schultz SK: Psychosis in late life: how does it fit into current diagnostic criteria? (case conference). Am J Psychiatry 1999; 156:624–629Abstract, Google Scholar
22. McClure FS, Gladsjo JA, Jeste DV: Late-onset psychosis: clinical, research, and ethical considerations (case conference). Am J Psychiatry 1999; 156:935–940Link, Google Scholar
23. Jeste DV, Harris MJ, Krull A, Kuck J, McAdams LA, Heaton R: Clinical and neuropsychological characteristics of patients with late-onset schizophrenia. Am J Psychiatry 1995; 152:722–730Link, Google Scholar
24. Howard R, Rabins PV, Seeman MV, Jeste DV, International Late-Onset Schizophrenia Group: Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international consensus. Am J Psychiatry 2000; 157:172–178Link, Google Scholar
25. Ciompi L: Catamnestic long-term study on the course of life and aging of schizophrenics. Schizophr Bull 1980; 6:606–618Crossref, Medline, Google Scholar
26. Harding CM, Brooks GW, Ashikaga T, Strauss JS, Landerl PD: Aging and social functioning in once-chronic schizophrenic patients 22–62 years after first admission: the Vermont story, in Schizophrenia and Aging. Edited by Miller NE, Cohen GD. New York, Guilford, 1987, pp 74–82Google Scholar
27. Ram R, Bromet EJ, Eaton WW, Pato C, Schwartz JE: The natural course of schizophrenia: a review of first-admission studies. Schizophr Bull 1992; 18:185–207Crossref, Medline, Google Scholar
28. Wyatt RJ: Neuroleptics and the natural course of schizophrenia. Schizophr Bull 1991; 17:325–351Crossref, Medline, Google Scholar
29. Harris MJ, Jeste DV: Late-onset schizophrenia: an overview. Schizophr Bull 1988; 14:39–55Crossref, Medline, Google Scholar
30. Kraepelin E: Dementia Praecox and Paraphrenia (1919). Translated by Barclay RM; edited by Robertson GM. New York, Robert E Krieger, 1971Google Scholar
31. Roth M: The natural history of mental disorder in old age. J Ment Sci 1955; 101:281–301>Crossref, Medline, Google Scholar



