Paroxetine in Human Breast Milk and Nursing Infants
Abstract
OBJECTIVE: The purpose of this study was to determine the extent of infant medication exposure through breast-feeding during maternal treatment with paroxetine. METHOD: Breast milk and paired maternal and infant sera were collected after 10 days of maternal treatment with paroxetine at a stable daily dose (10–50 mg/day). All samples were analyzed by means of high-performance liquid chromatography with ultraviolet detection and a limit of detection of 2 ng/ml. RESULTS: Breast milk paroxetine concentrations were highly variable (2–101 ng/ml) and were present in all breast milk samples (N=108). A significant gradient effect was observed, with greater paroxetine concentrations found in later portions of breast milk (hind milk) than in early portions (fore milk). No clear time course of paroxetine excretion into breast milk was demonstrated, although maternal paroxetine daily dose reliably predicted both trough and peak breast milk concentrations over a 24-hour period. In 16 mother and infant serum pairs, no detectable concentrations of paroxetine were found in the serum of the nursing infants. CONCLUSIONS: This study extends previous data by demonstrating the presence of paroxetine in the breast milk of nursing women treated with this medication. The low concentrations of paroxetine in infant serum and lack of any observable adverse effects after maternal use of this medication while breast-feeding parallels the available data on other selective serotonin reuptake inhibitors.
A previous comprehensive review highlighted the sparse data concerning the extent of infant exposure during lactation to selective serotonin reuptake inhibitors (SSRIs) (1). Case reports involving citalopram (2), fluoxetine (3–5), fluvoxamine (6), paroxetine (7), sertraline (8, 9), and venlafaxine (10) have been published. Other more extensive studies provided additional information on fluoxetine (11–13) and sertraline (14–16) treatment. The majority of these studies have reported either undetectable or very low serum SSRI concentrations in the breast-fed infants.
The complex pattern of SSRI excretion into breast milk, which demonstrates both gradient and time course effects, has been reported for fluoxetine (11), sertraline (15), and venlafaxine (10). These studies demonstrated the limitations of the milk-to-plasma ratio for the purpose of comparing infant exposure across different medications. Preliminary infant studies did not demonstrate the alterations in growth and weight curves (17) or infant platelet serotonin concentration (14) associated with sertraline exposure through breast milk. Furthermore, no alterations in neurodevelopment, as assessed by the Bayley and McCarthy Scale, were observed in four nursing infants exposed to fluoxetine (13). However, longer-term infant follow-up studies with larger sample sizes are necessary to assess the effects, if any, of infant exposure to SSRIs associated with lactation. The purpose of the current study was to characterize the breast milk excretion pattern and infant serum concentrations of paroxetine during maternal use of this medication while nursing.
METHOD
Subjects
Sixteen postpartum women being treated with paroxetine for major depression participated in the current study. Nine of the nursing women were treated at the Emory University Pregnancy and Postpartum Mood Disorders Program, and seven nursing women were followed at the Perinatal Psychiatry Program at Massachusetts General Hospital. Each subject and their partner were informed of other available treatment options (including psychotherapy, electroconvulsive therapy, or other antidepressants) and the unknown risks associated with nursing during treatment with paroxetine. All subjects who were participating in the current study requested infant serum monitoring as part of their clinical treatment plan. Written informed consent was obtained from all subjects for breast milk and maternal serum collection. All subjects were treated clinically, with dose adjustments made on the basis of side effects, symptom improvement, and syndrome resolution.
Sample Collection
All serum and breast milk samples were obtained after maternal serum paroxetine concentrations had attained steady state (patients were on a fixed-dose paroxetine regimen for longer than 10 days). Maternal and infant blood samples were collected in Vacutainer tubes without additives. Breast milk samples taken from the same breast were collected in sterile polypropylene tubes with the use of electric or manual breast pumps. Two different procedures were followed: 1) the initial 20 ml of breast milk samples were collected after maternal paroxetine dose at 4–6 hour intervals over a 24-hour period to determine the relationship between time after oral dose and breast milk concentration; 2) breast milk samples were collected in 10-ml aliquots from fore milk to hind milk to determine whether a concentration gradient for paroxetine excretion exists. All breast milk samples were labeled and stored in the patient’s home freezer until transfer to the laboratory. Once received at the Emory site, the samples were coded, stored at –80°C until assay, and analyzed blind to maternal daily dose of paroxetine.
Determination of Breast Milk and Serum Concentrations of Paroxetine
Breast milk sample analysis consisted of both a liquid/liquid and solid phase extraction (100-mg EXTRASEP C18, Nalge Nunc International, Rochester, N.Y.) followed by high-performance liquid chromatography separation and ultraviolet detection. Determination of paroxetine in serum requires only the solid phase extraction procedure. The quantification was accomplished through an isocratic high-performance liquid chromatography separation by using a 100 x 2-mm stainless steel reverse phase column, 3 µm, followed by ultraviolet detection at 225 nm. The analysis was performed with a model 1100 Hewlett Packard high-performance liquid chromatography chemstation equipped with a diode array detector. The mobile phase consisted of 0.02 M potassium phosphate monobasic, 110 µl N,N-demethyloctylamine/liter, 28% acetonitrile (pH 6.83). The flow rate of the mobile phase was set at 0.6 ml/min.
Calibration curves were constructed from medication-free human breast milk by the addition of varying amounts of paroxetine (0.0–500 ng). A five-point standard curve and two quality control specimens were included in each assay. The limit of detection was 2.0 ng/ml. Average correlation coefficients of variation were 3% intraassay and 8% interassay, at a concentration of 100 ng/ml.
Data Analysis
Breast milk concentration was divided by maternal serum concentration to provide the milk-to-plasma ratio for each aliquot of breast milk obtained. The effects of maternal daily dose on paroxetine breast milk concentrations were assessed with linear regression using minimum and maximum breast milk concentrations for each individual. To determine the excretion gradient of paroxetine into breast milk, the concentration for each fraction was divided by that of the minimum observed concentration [BMg]min (typically, the first 10-ml aliquot) and presented as a ratio from fore milk to hind milk. The time course was calculated in similar fashion by using the minimum breast milk concentration [BMt]min (typically 22–24 hours after maternal dose). To assess the total maximum daily infant dose, polynomial regression followed by integration to determine the area under the curve for breast milk gradients, time course, and correlation with maternal steady-state concentration were analyzed with the program Mathematica 2.2 (Wofram Research, Inc., Champaign, Ill.).
RESULTS
Sixteen postpartum women who were being treated with paroxetine at a mean once-a-day dose of 23.1 mg/day (SD=12) were included in the study. A total of 108 breast milk samples and 16 mother and infant serum pairs were obtained. Women submitted breast milk samples for both gradient and time course analysis, which accounted for 61 and 45 of the breast milk samples, respectively. Two samples were excluded from the final analysis as a result of incomplete labeling.
Detectable concentrations of paroxetine (2–101 ng/ml) were present in all breast milk samples (mean=41.6 ng/ml [SD=24.7]). Excretion gradient analysis of the breast milk paroxetine concentrations for each of the 11 subjects submitting breast milk samples (total N=61) revealed a significant volume/aliquot-dependent rate of excretion, with greater paroxetine concentrations in later aliquots of breast milk (hind milk). The data was best fit through third-order polynomial regression (r=0.41; F=3.80, df=3, 57, p=0.02) (figure 1).
The time course of excretion into breast milk for paroxetine, defined as the breast milk concentration at various time points after maternal dosage, was determined for eight women from whom more than three samples were collected in a 24-hour period (total N=45). Each sample obtained was the initial 10–20 ml of breast milk (fore milk) at each time point to control for the gradient effect. In contrast to our previous data with sertraline, a significant relationship with time course of excretion was not observed—third order polynomial (F=1.67, df=3, 41, p=0.19), data not shown. However, there was a significant relationship between maternal daily paroxetine dose and both trough (minimum) and peak (maximum) breast milk concentrations (figure 2).
The majority of the mother and infant serum pairs were obtained on the same day (N=14); the remaining two samples were collected within 7 days of each other because of logistical obstacles that precluded same-day sample collection. Maternal serum was obtained 2–5 hours after the daily paroxetine dose and infant serum samples were obtained 1–5 hours after nursing. Infant age and weight were variable across the group (mean=21.5 weeks [SD=16.3] and 6.6 kg [SD=1.9], respectively). The majority of infants (63%, N=10) were fully breast-fed and received no supplemental nutrition. All infant serum samples had undetectable concentrations of paroxetine (<2.0ng/ml). These data are summarized in table 1.
Upon direct interview, the parents were asked if they had noted any alteration in infant behavior, disposition, sleep, or activity or change in bowel movements after starting paroxetine treatment. In addition, parents were asked if the child had had regular pediatric visits, if the pediatrician had been informed of maternal paroxetine use, and if the pediatrician had made any comments concerning growth or infant development that warranted concern. No acute observable effects were reported for any of the 16 infants, although formal infant assessment was not performed.
DISCUSSION
The current study confirms and extends the breast-feeding literature by demonstrating that, similar to other antidepressants studied to date, paroxetine is excreted into human breast milk. The pattern of excretion is similar to those found in our previous studies that demonstrated higher concentrations in the more lipophilic hind milk. In contrast to our previous report with sertraline (15), no significant time course of paroxetine excretion into breast milk was demonstrated in the current study. Without determining a peak breast milk concentration of paroxetine over a 24-hour period, it is not possible to significantly predict the impact on infant dose by discarding a single breast-feeding. The reasons for the greater variability of the present data with paroxetine are unclear, but suggest that mathematical modeling of medication gradients and time course of excretion in breast milk may be complicated across different antidepressants. These data also demonstrate that the milk-to-plasma ratio of paroxetine can vary from 0.056 to 1.3, depending on the aliquot of breast milk assayed. Clearly the milk-to-plasma ratio can be affected both by portion of breast milk and timing of maternal serum sampling, and as such, this ratio may be of limited utility in estimating the infant daily dose for paroxetine. The intricate pattern of sertraline (15) and paroxetine excretion into the complex matrix of breast milk renders direct clinical application of breast milk analysis limited. Breast milk concentration analyses provide the basis (e.g., estimated dose) for interpretation of infant serum concentrations and a mechanism to potentially reduce infant exposure by discarding breast milk during peak concentrations.
This study confirms the conclusion of others (1) that the low concentrations of antidepressants and their metabolites that may be present in infant serum after exposure through nursing from mothers who use these agents require the use of research quality assays with well-documented lower limits of sensitivity. In contrast to reports on other SSRIs and tricyclic antidepressants, the infant serum concentrations were all below a more rigorously defined limit of detection (<2 ng/ml). These data were recently confirmed by Misri and colleagues (18), who also failed to find detectable concentrations of paroxetine in the serum of 25 breast-fed infants following analysis with gas chromatography and mass spectroscopy and a limit of detection of less than 1 ng/ml. Despite the inability to find detectable concentrations of paroxetine in infant serum, the consistent presence of paroxetine in breast milk demonstrates that the infant is exposed. The clinical significance of “trace” and “undetectable” infant serum concentrations is unknown but underscores the need to cautiously interpret laboratory results described as “undetectable.” The reporting of “0 ng/ml” in infant serum (16, 19) could be misinterpreted by clinicians as suggesting a complete absence of infant exposure to medication. Even with the capacity to more accurately quantify infant medication exposure through nursing, limited lactation-specific laboratory data preclude any conclusions about potential sequestration in more lipophilic infant tissues such as the central nervous system. Given the high degree of variation in monoamine transporter and receptor affinity for the individual SSRIs (20–22), definitive conclusions based on “absolute exposure” (ng/ml) without consideration of the potential “functional exposure” (percent receptor/transporter occupancy) that takes into account such variability in transporter and receptor affinities is premature.
It is somewhat reassuring that in the current study no acute adverse events in the infants were reported by the parents or by pediatricians. However, the clinical significance of chronic SSRI exposure on neurobehavioral development remains unknown and is an important area of future inquiry.
CONCLUSIONS
The current study provides the first detailed characterization of paroxetine excretion into human breast milk and further extends the rapidly accumulating data on the relative safety of these medications during lactation. These data demonstrate the complexity of the pharmacokinetics of medication excretion into breast milk and the extent of infant exposure based on measurement of infant serum. This study is unique in its sample size and the collaborative effort to standardize the methodology in assay and data collection techniques, which may otherwise make interpretation of data difficult. Data that quantify infant exposure help to define the risk/benefit assessment for puerperal women who must weigh the relative risks of infant exposure to psychiatric medication use during lactation versus the risks of the untreated maternal psychiatric illness.
Received Feb. 10, 1999; revision received June 10, 1999; accepted July 12, 1999. From the Department of Psychiatry and Behavioral Sciences, the Department of Gynecology and Obstetrics, and the Department of Pathology, Emory University School of Medicine; and the Department of Psychiatry, Harvard University School of Medicine, Cambridge, Mass. Address reprint requests to Dr. Stowe, Pregnancy and Postpartum Mood Disorders Program, Emory University School of Medicine, 1639 Pierce Dr., Suite 4003, Atlanta, GA 30322. Supported in part by NIMH grant MH-51761, unrestricted educational grants from SmithKline Beecham Pharmaceuticals and Pfizer Inc, and an APA/SmithKline Beecham Young Faculty Award (Dr. Stowe).
 |
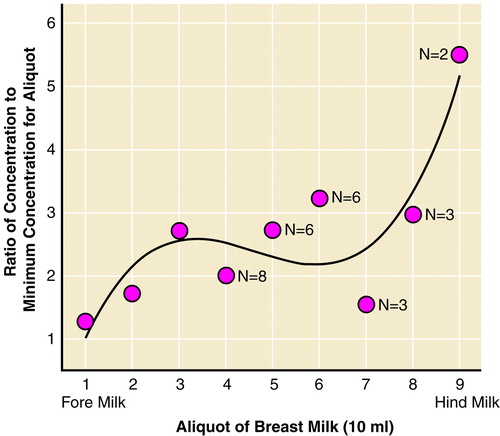
FIGURE 1. Mean Paroxetine Concentrations From Early to Later Portions of Breast Milk From 61 Samples Obtained From 11 Womena
aData represent 61 breast milk samples collected 8–12 hours after maternal oral daily dose of paroxetine. Each woman submitted three or more breast milk samples for determination of gradient effects from fore milk to hind milk. Paroxetine concentrations increased from the initial portion of breast milk to the later portions of breast milk. The initial three 10-ml aliquots were obtained from all 11 women. It is feasible that the diminishing cohort size after the first 40 ml of breast milk altered the best-fit polynomial equation.
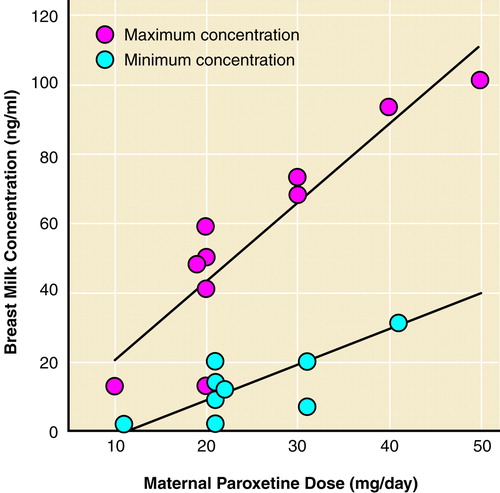
FIGURE 2. Effects of Maternal Paroxetine Dose on Minimum and Maximum Paroxetine Breast Milk Concentrationsa
aMaternal paroxetine dose was significantly correlated with both the minimum (r=0.88, df=43, p<0.001) and maximum (r=0.90, df=43, p<0.001) breast milk concentrations.
1. Wisner KL, Perel JM, Findling RL: Antidepressant treatment during breast-feeding. Am J Psychiatry 1996; 153:1132–1137Google Scholar
2. Jensen PN, Olesen OV, Bertelsen A, Linnet K: Citalopram and desmethylcitalopram in breast milk and in serum of mother and infant. Ther Drug Monit 1997; 19:236–239Crossref, Medline, Google Scholar
3. Burch KJ, Wells BG: Fluoxetine/norfluoxetine concentrations in human milk. Pediatrics 1992; 89:676–677Medline, Google Scholar
4. Isenberg KE: Excretion of fluoxetine in human breast milk (letter). J Clin Psychiatry 1990; 51:169Medline, Google Scholar
5. Lester BM, Cucca J, Andreozzi L, Flanagan P, Oh W: Possible association between fluoxetine hydrochloride and colic in an infant. J Am Acad Child Adolesc Psychiatry 1993; 32:1253–1255Google Scholar
6. Wright S, Dawling S, Ashford JJ: Excretion of fluvoxamine in breast milk. Br J Clin Pharmacol 1991; 31:209Crossref, Medline, Google Scholar
7. Spigset O, Carleborg L, Norstrom A, Sandlund M: Paroxetine level in breast milk (letter). J Clin Psychiatry 1996; 57:39Medline, Google Scholar
8. Altshuler LL, Burt VK, McMullen M, Hendrick V: Breastfeeding and sertraline: a 24 hour analysis. J Clin Psychiatry 1995; 56:243–245Medline, Google Scholar
9. Mammen OK, Perel JM, Rudolph G, Foglia JP, Wheeler SB: Sertraline and norsertraline levels in three breastfed infants. J Clin Psychiatry 1997; 58:100–103Crossref, Medline, Google Scholar
10. Ilett KF, Hackett LP, Dusci LJ, Roberts MJ, Kristensen JH, Paech M, Groves A, Yapp P: Distribution and excretion of venlafaxine and O-desmethylvenlafaxine in human milk. Br J Clin Pharmacol 1998:45:459–462Google Scholar
11. Taddio A, Ito S, Koren G: Excretion of fluoxetine and its metabolite, norfluoxetine, in human breast milk. J Clin Pharmacol 1996; 36:42–47Crossref, Medline, Google Scholar
12. Kim J, Misri S, Riggs KW, Ryan DM, Carter D, Rurak DW: Stereoselective excretion of fluoxetine and norfluoxetine in breast milk and neonatal exposure, in 1997 Annual Meeting New Research Program and Abstracts. Washington, DC, American Psychiatric Association, 1997, pp 127–128Google Scholar
13. Yoshida K, Smith B, Craggs M, Kumar RC: Fluoxetine in breast-milk and developmental outcome of breast-fed infants. Br J Psychiatry 1998; 172:175–178Crossref, Medline, Google Scholar
14. Eppersen CN, Anderson GM, McDougle CJ: Sertraline and breast-feeding (letter). N Engl J Med 1997; 336:1189–1190Google Scholar
15. Stowe ZN, Owens MJ, Landry JC, Kilts CD, Ely T, Llewellyn A, Nemeroff CB: Sertraline and desmethylsertraline in human breast milk and nursing infants. Am J Psychiatry 1997; 154:1255–1260Google Scholar
16. Wisner KL, Perel JM, Blumer J: Serum sertraline and N-desmethylsertraline levels in breast-feeding mother-infant pairs. Am J Psychiatry 1998; 155:690–692Link, Google Scholar
17. Llewellyn AM, Stowe ZN, Nemeroff CB: Infant outcome after sertraline exposure, in 1997 Annual Meeting New Research Program and Abstracts. Washington, DC, American Psychiatric Association, 1997, p 176Google Scholar
18. Misri S, Carter D, Ryan DM: Paroxetine levels in mother/infant dyad, in 1999 Annual Meeting Syllabus and Proceedings Summary. Washington, DC, American Psychiatric Association, 1999, p 100Google Scholar
19. Wisner KL, Perel JM, Findling RL, Hinnes RL: Nortriptyline and its hydroxymetabolites in breastfeeding mothers and newborns. Psychopharmacol Bull 1997; 33:249–251Medline, Google Scholar
20. Owens MJ, Morgan WN, Plott SJ, Nemeroff CB: Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther 1997; 283:1305–1322Google Scholar
21. Bolden-Watson C, Richelson E: Blockade by newly-developed antidepressants of biogenic amine uptake into rat brain synaptosomes. Life Sci 1993; 52:1023–1029Google Scholar
22. Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology 1994; 114:559–565Crossref, Medline, Google Scholar



