Support for Allelic Association of a Polymorphic Site in the Promoter Region of the Serotonin Transporter Gene With Risk for Alcohol Dependence
Abstract
OBJECTIVE: An association between the 5-HTTLPR short variant polymorphism in the promoter region of the serotonin transporter gene and risk for alcohol dependence has been reported from case-control studies that are, however, prone to chance findings related to artifacts of population structure. The authors sought additional evidence for this association from a family-based study. METHOD: Ninety-two alcohol-dependent probands and their parents were tested for nonrandom transmission of alleles from heterozygous parents to affected probands. RESULTS: Preferential transmission of the short allele was found (65 of 102 transmissions from heterozygous parents). CONCLUSIONS: The results suggest allelic association between a variant in the promoter region of the serotonin transporter gene and the risk for alcohol dependence. However, it remains to be seen whether the functional properties of this variant are directly responsible for the increased risk to alcohol dependence.
Studies in rodents and humans have pointed to a relationship between low levels of brain serotonin turnover and high levels of alcohol intake that may play a crucial role in the initiation and maintenance of alcoholism. Decreased availability of the serotonin transporter to radioligands has been found in brain imaging studies of probands with alcohol dependence (1) and in postmortem studies of human brains after alcohol exposure (2). Thus, a polymorphism in the promoter region of the serotonin transporter gene (3) can be considered a good candidate for conferring genetic susceptibility to alcohol dependence. It has been shown that the two common variants of this polymorphic site differ in the effect they have on the transcriptional activity of the gene in vitro (3), although it is unclear which of several activators or silencers of transcription (4) is to be held accountable. Such functional polymorphisms are particularly valuable in tests for association with complex traits such as alcohol dependence. The traditional approach is to compare allele frequencies between afflicted individuals and unrelated matched healthy comparison subjects. In studies using this design, a higher frequency of the short allele has been found in probands with alcohol dependence (5). Other studies have demonstrated an association between the short allele and increased neuroticism (3), a personality trait that in turn figures as a risk factor for alcoholism (6). Since case-control studies are prone to false-positive results caused by population structure (7), we used a family-based study design to investigate the association between risk for alcohol dependence and the 5-HTTLPR polymorphism in the promoter region of the serotonin transporter gene.
Method
Ninety-two inpatients (mean age=35.3 years, SD=6.3, 82.6% of whom were male) from the alcohol detoxification programs of two psychiatric university hospitals in Germany and Hungary and their parents donated blood samples and were interviewed with the Semi-Structured Assessment for the Genetics of Alcoholism (8) after receiving a complete description of the aims and the procedures of the study and after having given written informed consent. Inpatient probands fulfilled DSM-IV criteria for alcohol dependence at a mean age of 26.6 years (SD=7.1). Antisocial personality traits were present in 10 probands (10.9%) and a history of delirium in 18 probands (19.6%) and of withdrawal seizures in 24 probands (26.1%).
Genotyping of DNA samples was performed by polymerase chain reaction, as described elsewhere (9). Fragments of 484 or 528 base pairs corresponding to the short and long alleles, respectively, were separated by agarose gel electrophoresis and visualized by ethidium bromide staining.
Under the null hypothesis of no association, the short and the long alleles from heterozygous parents (genotype short/long) would be transmitted to affected probands at equal frequency. Association as indicated by departure from random transmission can thus be identified with the Transmission/Disequilibrium Test (7), a goodness-of-fit chi-square test with one degree of freedom.
Results
All 276 individuals in the 92 parent-offspring triads were genotyped for the 5-HTTLPR polymorphism. Information for the Transmission/Disequilibrium Test was provided only by 74 triads with at least one heterozygous parent; 18 triads in which both parents were homozygous did not contribute. Of the total of 184 parental transmissions, 102 were from heterozygous parents and thus were informative for the Transmission/Disequilibrium Test analysis. The short allele was transmitted 65 times and not transmitted 37 times. This difference from equal distribution was statistically significant (Transmission/Disequilibrium Test: χ2=7.69, df=1, p<0.006).
Discussion
The study results provide support for allelic association of the 5-HTTLPR short variant with alcohol dependence. To our knowledge, this is the first report suggesting association by using a family-based design, which avoids confounding factors of population structure as they may be present in case-control studies. Association of the short allele with risk for alcohol dependence has been reported from case-control studies (5, 10, 11), some restricted to probands who had withdrawal seizures or delirium (5) or dissocial personality (10), although two other case-control studies (12, 13) and a family-based study (14) failed to confirm this association.
An association of the 5-HTTLPR variant with alcohol dependence could have significant functional consequences. The short allele has been shown to result in less transporter gene transcription than the long one (3, 15) and, consequently, in a lower density of transporter ligand binding sites in postmortem brains (15). The association we found is therefore consistent with the observation that central serotonin transporter availability to ligands is lower in alcohol-dependent probands after detoxification (1) and in postmortem brains of subjects who had been exposed to alcohol (2) than in comparison subjects. However, the link between lower basal transcriptional activity associated with the short allele and a higher risk for alcohol dependence remains to be shown. One mechanism might be that chronic alcohol use in individuals with a short allele, but not in those with two long alleles, seems to lead to an up-regulation of basically lower transporter density (15) (Figure 1).
Despite the superior properties of the Transmission/Disequilibrium Test, a false positive result remains a possibility. Therefore, replication studies, preferably family-based, in different populations are required. Although favorable treatment response may not necessarily be associated with the same allele as disease susceptibility, our finding, if confirmed, should encourage further studies to explore whether the currently limited benefits of alcoholism treatment with serotonergic drugs could be increased by specifically providing these medications to individuals with genetic vulnerability as indicated by the presence of the 5-HTTLPR short variant.
Received Aug. 19, 1999; revisions received March 1 and May 5, 2000; accepted June 2, 2000. From the Department of Psychiatry and Medical Psychology, University Medical School of Pécs, Hungary; Division of Molecular Genetics, Rudjer Boković Institute, Zagreb, Croatia; the Institute for Medical Statistics and the Department of Psychiatry, University of Bonn. Address reprint requests to Dr. Lichtermann, Department of Psychiatry, University of Bonn, Sigmund-Freud-Str 25, D-53105 Bonn, Germany; [email protected] (e-mail). Supported by grant 01 EB 94 18 from the Federal Ministry of Education, Science, Research and Technology, Germany; grant 111/38 from the BONFOR Research Commission of the Medical Faculty at the University of Bonn; and a grant from the Ministry of Women, Youth, Family and Health of the State of Nordrhein-Westfalen, Germany. The authors thank the patients and their relatives for their contributions.
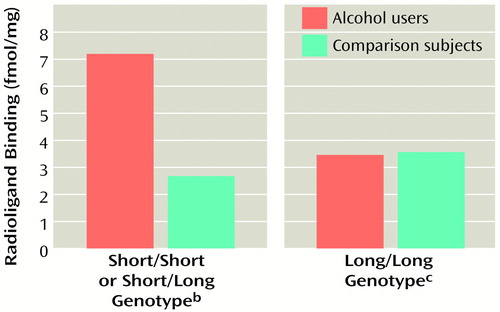
Figure 1. Radioligand Binding to Midbrain Serotonin Transporter in Postmortem Brains of Alcohol Users and Comparison Subjects, by Genotype of the 5-HTTLPR Polymorphisma
aData from Little et al. (15). Significant difference between alcohol users with the short/short or short/long genotype and comparison subjects (t=2.97, df=25, p=0.006, two-tailed).
bN=7 for alcohol users; N=9 for comparison subjects.
cN=5 for alcohol users; N=9 for comparison subjects.
1. Heinz A, Ragan P, Jones DW, Hommer D, Williams W, Knable MB, Gorey JG, Doty L, Geyer C, Lee KS, Coppola R, Weinberger DR, Linnoila M: Reduced central serotonin transporters in alcoholism. Am J Psychiatry 1998; 155:1544–1549Google Scholar
2. Gross-Isseroff R, Biegon A: Autoradiographic analysis of [3H]imipramine binding in the human brain postmortem: effects of age and alcohol. J Neurochem 1988; 51:528–534Crossref, Medline, Google Scholar
3. Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL: Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996; 274:1527–1531Google Scholar
4. Mortensen OV, Thomassen M, Larsen MB, Whittemore SR, Wiborg O: Functional analysis of a novel human serotonin transporter gene promoter in immortalized raphe cells. Brain Res Mol Brain Res 1999; 68:141–148Crossref, Medline, Google Scholar
5. Sander T, Harms H, Lesch KP, Dufeu P, Kuhn S, Hoehe M, Rommelspacher H, Schmidt LG: Association analysis of a regulatory variation of the serotonin transporter gene with severe alcohol dependence. Alcohol Clin Exp Res 1997; 21:1356–1359Google Scholar
6. Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG: Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol Med 1997; 27:1381–1396Google Scholar
7. Spielman RS, Ewens WJ: The TDT and other family-based tests for linkage disequilibrium and association. Am J Hum Genet 1996; 59:983–989Medline, Google Scholar
8. Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI Jr, Reich T, Schmidt I, Schuckit MA: A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol 1994; 55:149–158Crossref, Medline, Google Scholar
9. Hranilovic D, Schwab SG, Jernej B, Knapp M, Lerer B, Albus M, Rietschel M, Kanyas K, Borrmann M, Lichtermann D, Maier W, Wildenauer DB: Serotonin transporter gene and schizophrenia: evidence for association/linkage disequilibrium in families with affected siblings. Mol Psychiatry 2000; 5:91–95Crossref, Medline, Google Scholar
10. Sander T, Harms H, Dufeu P, Kuhn S, Hoehe M, Lesch KP, Rommelspacher H, Schmidt LG: Serotonin transporter gene variants in alcohol-dependent subjects with dissocial personality disorder. Biol Psychiatry 1998; 43:908–912Crossref, Medline, Google Scholar
11. Hammoumi S, Payen A, Favre JD, Balmes JL, Benard JY, Husson M, Ferrand JP, Martin JP, Daoust M: Does the short variant of the serotonin transporter linked polymorphic region constitute a marker of alcohol dependence? Alcohol 1999; 17:107–112Google Scholar
12. Gelernter J, Kranzler H, Cubells JF: Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African- and European-American and Japanese populations and in alcohol-dependent subjects. Hum Genet 1997; 101:243–246Crossref, Medline, Google Scholar
13. Jorm AF, Henderson AS, Jacomb PA, Christensen H, Korten AE, Rodgers B, Tan X, Easteal S: An association study of a functional polymorphism of the serotonin transporter gene with personality and psychiatric symptoms. Mol Psychiatry 1998; 3:449–451Crossref, Medline, Google Scholar
14. Edenberg HJ, Reynolds J, Koller DL, Begleiter H, Bucholz KK, Conneally PM, Crowe R, Goate A, Hesselbrock V, Li TK, Nurnberger JI Jr, Porjesz B, Reich T, Rice JP, Schuckit M, Tischfield JA, Foroud T: A family-based analysis of whether the functional promoter alleles of the serotonin transporter gene HTT affect the risk for alcohol dependence. Alcohol Clin Exp Res 1998; 22:1080–1085Google Scholar
15. Little KY, McLaughlin DP, Zhang L, Livermore CS, Dalack GW, McFinton PR, DelProposto ZS, Hill E, Cassin BJ, Watson SJ, Cook EH: Cocaine, ethanol, and genotype effects on human midbrain serotonin transporter binding sites and mRNA levels. Am J Psychiatry 1998; 155:207–213Link, Google Scholar



