Nicotine Transdermal Patch and Atypical Antipsychotic Medications for Smoking Cessation in Schizophrenia
Abstract
OBJECTIVE: Schizophrenic patients have high rates of cigarette smoking. The authors compared the outcomes of two group psychotherapy programs for smoking cessation in patients with schizophrenia or schizoaffective disorder who were also treated with the nicotine transdermal patch and with either atypical or typical antipsychotic medications. METHOD: Forty-five subjects were randomly assigned to 1) the group therapy program of the American Lung Association (N=17) or 2) a specialized group therapy program for smokers with schizophrenia (N=28) that emphasized motivational enhancement, relapse prevention, social skills training, and psychoeducation. All subjects participated in 10 weeks of treatment with the nicotine transdermal patch (21 mg/day) and 10 weekly group therapy sessions and continued to receive their prestudy atypical (N=18) or typical (N=27) antipsychotic medications. Outcome variables included treatment retention, rate of smoking abstinence, and expired-breath carbon monoxide level. RESULTS: Smoking abstinence rates did not differ in the two group therapy programs. However, atypical antipsychotic agents, in combination with the nicotine transdermal patch, significantly enhanced the rate of smoking cessation (55.6% in the atypical agent group versus 22.2% in the typical group), which was reflected by a significant effect of atypical versus typical agents on carbon monoxide levels. Risperidone and olanzapine were associated with the highest quit rates. CONCLUSIONS: The results suggest that 1) smoking cessation rates with the nicotine transdermal patch are modest in schizophrenia, 2) specialized group therapy for schizophrenic patients is not significantly different from American Lung Association group therapy in its effect on smoking cessation, and 3) atypical agents may be superior to typical agents in combination with the nicotine transdermal patch for smoking cessation in schizophrenia.
Schizophrenic patients have higher rates of nicotine dependence through cigarette smoking (58%–88%), compared with the general population (about 25%) (1), and are often heavily dependent smokers who have great difficulty with smoking cessation (2–4). Low intrinsic motivation to quit smoking, which may be related to negative and affective symptoms characteristic of schizophrenic illness, has been proposed as a factor for the continued high rates of cigarette smoking in schizophrenic patients and their difficulties with smoking cessation (2, 5, 6). Schizophrenic subjects tend to be in the early stages of motivation to quit smoking, as assessed by a model of change of smoking behavior (5, 7). As subjects with schizophrenia are at high risk for developing medical morbidity and mortality related to chronic smoking (8, 9), helping schizophrenic smokers to quit smoking is an important undertaking. Few studies of smoking cessation in schizophrenic patients have been reported (2, 10), with modest endpoint smoking abstinence rates in patients compared to nonpsychiatric smokers.
A number of potential interventions could improve outcomes for smoking cessation/reduction in schizophrenic patients. Several preliminary studies have suggested that the atypical antipsychotic agent clozapine may reduce smoking consumption in schizophrenic smokers (11–13), particularly those who are heavily dependent smokers (12). In fact, one study suggested that the typical antipsychotic agent haloperidol could increase cigarette smoking (14). A case report has suggested that the FDA-approved antidepressant agent, bupropion, sustained release, may enhance smoking cessation efforts in schizophrenic patients (15), and preliminary placebo-controlled studies have supported the efficacy of sustained-release bupropion versus placebo in reducing cigarette smoking in this patient group (16, 17).
Few smoking cessation programs have been targeted toward psychiatric patients, particularly those with schizophrenic disorders. Addington et al. (10) have reported use of a modified version of the 7-week behavioral program offered by the American Lung Association Freedom From Smoking Program. This group therapy program emphasized psychoeducation, positive reinforcement, anxiety reduction, and adjunctive use of the nicotine transdermal patch (18); the endpoint smoking abstinence rate in Addington et al.’s study was 42% (10). Behavioral interventions such as contingency management may also improve smoking cessation outcomes in opiate-dependent smokers (19, 20) and in smokers with schizophrenia (21). Preliminary work from our group (2) has suggested that combining optimal schizophrenia interventions (e.g., psychoeducation, social skills training) with smoking cessation interventions (e.g., motivational enhancement therapy, relapse prevention therapy) may improve treatment outcomes and tolerability of treatment.
In the study reported here, our primary goal was to compare the American Lung Association’s behavioral program to a manualized smoking cessation treatment program designed for patients with schizophrenia during a 12-week trial in nicotine-dependent schizophrenic smokers who were motivated to quit smoking. A secondary goal was to examine the effects of antipsychotic treatment status (atypical versus typical agents) in combination with the nicotine patch on smoking cessation outcomes.
Method
A total of 58 subjects who met DSM-IV criteria for schizophrenia or schizoaffective disorder and nicotine dependence were screened for this study. Forty-five eligible subjects gave their written consent to participate and were randomly assigned to study groups. The protocol was approved by the human investigation committee of Yale University School of Medicine.
All subjects were evaluated at baseline with the Structured Clinical Interview for DSM-IV, the Positive and Negative Syndrome Scale (22), the Beck Depression Inventory (23), the Shiffman-Jarvik Nicotine Withdrawal Scale (24), the Webster Extrapyramidal Symptoms Scale (25), the Abnormal Involuntary Movement Scale (26), and the Fagerstrom Test for Nicotine Dependence (27). Expired-breath carbon monoxide was measured in ppm from a single breath by using a carbon monoxide monitor (Vitalograph, Inc., Lenexa, Kan.). To be included in the study, subjects required a score of 5 or higher on the Fagerstrom Test for Nicotine Dependence.
Eligible subjects (N=45) were assigned to groups by using a block randomization procedure such that when four to six subjects were screened and considered eligible for study participation, they were assigned together to either the American Lung Association program or the specialized schizophrenia group therapy treatments. Weekly group therapy sessions were conducted for a total of 10 weeks. The American Lung Association group participated in a standard 7-week manualized behavioral group therapy program (28–30) and were seen for supportive group counseling during the remaining three weekly group sessions. Each session lasted 60 minutes. The specialized schizophrenia smoking cessation program, described previously (2), included 3 weeks of motivational enhancement therapy (31) (weeks 1 through 3) and seven weeks of psychoeducation (18), social skills training, and relapse prevention strategies (2) (weeks 4 through 10). The smoking “quit date” occurred during week 3 of both group therapy programs. At this time, all subjects began wearing the 24-hour nicotine transdermal patch (21 mg/day) for a total of 6 weeks. Subsequently, nicotine patch dose was tapered (14 mg in weeks 9 and 10; 7 mg in weeks 11 and 12), and then the nicotine patch was discontinued. Smoking abstinence was determined by self-reported cigarette use and verified with a carbon monoxide level <10 ppm. Antipsychotic medications were maintained at the prestudy dose for the duration of the study; subjects who required a dose change for symptom stabilization or antipsychotic side effects were excluded from the analysis. Subjects were followed up at 6 months after study completion to determine point-prevalence smoking abstinence rates.
Kaplan-Meier survival analysis was used to determine differences in retention rates between the two psychotherapy treatment groups (controlling for medication treatment) and between the two medication groups (controlling for group therapy assignment) (32). Chi-square tests were used to analyze smoking cessation outcome data. Subjects who were lost to 6-month follow-up were counted as smokers. Hierarchical linear modeling (33, 34) was used to determine whether the linear rate of change across the course of the study in continuous outcome measures (e.g., carbon monoxide levels) varied as a function of treatment group (a randomization factor) and antipsychotic medication (a subject factor). Statistical analysis was done by using Microsoft Excel 2000 for descriptive statistics and chi-square analysis, SPSS version 8.0 for survival analysis, and MIXREG software for hierarchical linear modeling analyses. Post hoc differences were considered significant when p<0.05.
Results
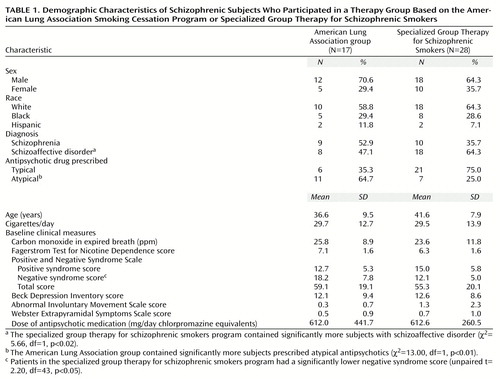
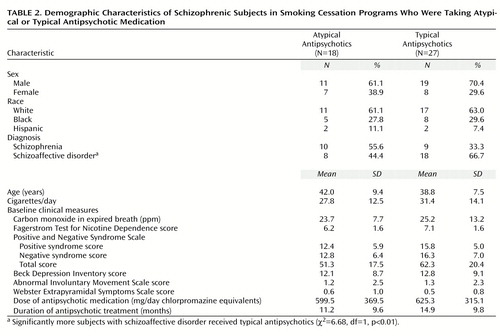
The demographic characteristics of subjects assigned to the American Lung Association group and the specialized group therapy program for schizophrenic smokers are presented in Table 1. Approximately 66.7% of subjects were male, 62.2% were Caucasian, 28.9% were African American, and 8.9% were Hispanic. A total of 42.2% of subjects met criteria for a DSM-IV diagnosis of schizophrenia. The specialized group therapy program for schizophrenic smokers included significantly more subjects with schizoaffective disorder than the American Lung Association group. A greater proportion of subjects were prescribed atypical antipsychotic medications in the American Lung Association group than in the specialized group therapy program. Subjects smoked an average of about 30 cigarettes per day, had a mean baseline expired-breath carbon monoxide level of approximately 25 ppm, and a mean Fagerstrom Test for Nicotine Dependence score of approximately 6.5, indicating moderate levels of nicotine dependence. Scores on the Positive and Negative Syndrome Scale indicated a modest level of psychotic illness; the level of negative symptoms was significantly lower in the specialized group therapy patients. Beck Depression Inventory scores suggested a mild degree of affective symptoms, consistent with the observation that 57.8% of subjects had a diagnosis of schizoaffective disorder. Dyskinetic and extrapyramidal side effect scores were minimal; 40.0% of subjects (18 of 45) were treated with an atypical antipsychotic agent. The mean antipsychotic exposure (in chlorpromazine equivalents [35]) was approximately 600 mg/day. With the exception of a significant difference in the proportions of subjects with schizoaffective disorder, there were no differences on baseline characteristics between subjects who received typical antipsychotic agents and those who received atypical antipsychotics (Table 2).
Findings for treatment retention, as assessed by Kaplan-Meier survival analysis, are presented in Figure 1. Survival analysis indicated that the difference in reduction in subject retention in the two therapy groups during the last 6 weeks of the trial was nonsignificant (log rank test=0.87, df=1, p=0.35) (Figure 1, upper panel). There were, however, significant differences in treatment retention between subjects treated with typical versus atypical antipsychotics (log rank test=5.03, df=1, p<0.05), with significant attrition by trial endpoint among subjects who received typical antipsychotics (Figure 1, lower panel). Total weeks in treatment during the trial were significantly reduced in the typical versus atypical antipsychotic treatment groups (typical: mean=7.3 weeks, SD=4.2; atypical: mean=10.3 weeks, SD=3.0) (unpaired t test, t=3.04, df=43, p<0.05).
Rates of smoking abstinence at the trial endpoint and at 6-month follow-up are presented in Figure 2. Smoking abstinence rates at endpoint did not differ significantly between the American Lung Association group (six of 17 subjects, 35.3%) and the specialized group therapy patients (10 of 28 subjects, 35.7%) (χ2=0.16, df=1, p=0.69). However, when subjects were stratified by antipsychotic class (typical versus atypical antipsychotic), there was a significant effect on smoking abstinence rates of atypical (10 of 18 subjects; 55.6%) versus typical (six of 27 subjects; 22.2%) antipsychotic use, in combination with the nicotine transdermal patch (χ2=22.75, df=1, p<0.01), with atypical antipsychotic treatment more than doubling the abstinence rate. Rates of continuous smoking abstinence in the last 4 weeks of treatment were 44.4% (eight of 18 subjects) of those taking atypical agents versus 18.5% (five of 27 subjects) of those taking typical agents (χ2=15.53, df=1, p<0.01) and 32.1% (nine of 28 subjects) of the specialized group therapy patients versus 23.5% (four of 17 subjects) of those in the American Lung Association group (χ2=3.67, df=1, p=0.06). The findings suggest that the differences between atypical and typical antipsychotic groups were also significant on this abstinence measure. Six-month smoking abstinence rates were considerably lower than those at trial endpoint (Figure 2). This result was due in part to a considerable loss of study subjects by the time of the follow-up assessments. Smoking abstinence rates at 6-month follow-up were 17.6% (three of 17) of those in the American Lung Association group versus 10.7% (three of 28) of the specialized group therapy patients (χ2=4.84, df=1, p<0.03) and 16.7% (three of 18) of those taking atypical antipsychotics versus 7.4% (two of 27) of those taking typical antipsychotics (χ2=7.00, df=1, p<0.02). The differences favored the American Lung Association group and the group treated with atypical antipsychotics. Differences in smoking abstinence rates between the groups taking typical and atypical agents were not accounted for by the higher proportion of subjects with schizoaffective disorder in the typical antipsychotic group (Table 2). The smoking abstinence rate for subjects with schizoaffective disorder (five of seven subjects, 71.4%) was not significantly different from the rate for patients with schizophrenia (six of 11 subjects, 54.5%) in the atypical antipsychotic group (χ2=1.97, df=1. p=0.15) and were higher for subjects with schizoaffective disorder (seven of 18 subjects, 38.9%) than for subjects with schizophrenia (three of nine subjects, 33.3%) in the typical antipsychotic group (χ2=7.13, df=1, p<0.01). Furthermore, the differences in overall smoking abstinence rates between subjects with schizoaffective disorder (10 of 24 subjects, 41.7%) and subjects with schizophrenia (eight of 21 subjects, 38.1%) were not significant (χ2=1.43, df=1, p=0.24).
There were no significant differences in psychiatric symptoms or medication side effects between the American Lung Association group and the specialized group therapy patients or between subjects who received typical versus atypical antipsychotic medications (data not shown). Furthermore, levels of dyskinetic and extrapyramidal symptoms did not significantly differ between abstainers and those who continued to smoke (data not shown). The dose of adjunctive anticholinergic medication prescribed in the typical antipsychotic group (mean=1.0 mg/day, SD=0.6, expressed in benztropine equivalents [35]) was significantly higher than in the atypical antipsychotic group (mean=0.3 mg/day, SD=0.5) (t=4.74, df=43, p<0.01, unpaired t test). The psychological symptoms of nicotine abstinence, as assessed by the psychological subscale of the Shiffman-Jarvik Nicotine Withdrawal Scale, increased significantly in quitting versus nonquitting smokers (t=2.65, df=23, p<0.01, unpaired t test) at week 4 (1 week after the quit date), but hierarchical linear modeling analysis did not reveal a significant interaction of smoking status and time during the trial (z=–1.43, p=0.15).
Figure 3 presents data on the effects of group treatment (American Lung Association group versus the specialized group therapy patients) and antipsychotic treatment (atypical versus typical agents) on levels of expired-breath carbon monoxide. Hierarchical linear modeling analysis of weekly carbon monoxide levels revealed a significant interaction of medication and time (z=–2.19, p<0.03), and there was a reduction in carbon monoxide levels after application of the nicotine patch (weeks 3–12) in patients treated with atypical versus typical antipsychotic agents (Figure 3). No evidence of an interaction of group and time was observed for carbon monoxide levels in the comparison of the American Lung Association group and the specialized group therapy patients (z=–0.20, p=0.84).
Smoking cessation rates of subjects grouped by the specific antipsychotic agent they received during the trial are shown in Figure 4. Among subjects who received atypical agents, those with the highest smoking cessation rates were those who received risperidone (three of five subjects, 60.0%) and olanzapine (five of seven subjects, 71.4%), but the group sizes were small. The atypical agents prescribed for study subjects and their mean daily doses were clozapine, mean=285.0 mg/day, SD=129.0; risperidone, mean=4.0 mg/day, SD=1.8; olanzapine, mean=11.1 mg/day, SD=2.9; and quetiapine, mean=400.0 mg/day, SD=81.6. The typical agents and their mean daily doses were perphenazine, mean=34.7 mg/day, SD=12.1; fluphenazine, mean=17.1 mg/day, SD=10.9; haloperidol, mean=10.4 mg/day, SD=2.4; chlorpromazine, mean=775.0 mg/day, SD=225.0; and thiothixene, mean=22.5 mg/day, SD=0.0. Rates of smoking cessation in subjects treated with typical antipsychotics were generally much lower (about 20%–30%) than in subjects treated with atypical agents (Figure 4).
Discussion
The major finding of this study was that schizophrenic subjects who were treated with atypical versus typical antipsychotic medications in combination with the nicotine transdermal patch had enhanced smoking cessation rates. In fact, endpoint smoking cessation rates in patients who received atypical agents approached those observed in studies of the effects of the nicotine transdermal patch in smokers who were not psychiatric patients (36). Our results add to the growing impression that medications that target specific clinical symptoms (e.g., negative symptoms, cognitive deficits, reduction of extrapyramidal symptoms) and neurochemical aspects (e.g., cortical dopaminergic hypofunction) of schizophrenic illness may produce improvements in a number of domains, including psychopathology and drug dependence. Several anecdotal reports have suggested that atypical antipsychotics may reduce substance use in schizophrenic drug abusers (37, 38), and at least three studies have found that the atypical antipsychotic clozapine may reduce smoking consumption in schizophrenic smokers who are switched to this agent (11–13). Our data on subjects grouped by antipsychotic class also suggest a differential effect of various atypical agents for improving smoking abstinence rates in combination with the nicotine transdermal patch. Risperidone and olanzapine appear superior in this regard, although our findings are limited by the small size of the study groups. The substantial reduction in smoking abstinence rates in both the atypical and typical groups at 6-month follow-up suggests that these effects are not durable after discontinuation of the nicotine transdermal patch, even though the superiority of atypical versus typical agents persisted. Atypical antipsychotic agents are known to improve extrapyramidal side effects and akathisia, negative symptoms, and various domains of neuropsychological function, as well as to improve deficient sensory physiology in animals (39) and in human subjects (40, 41). Future investigations should evaluate the effectiveness of atypical versus typical agents as adjuncts for smoking cessation in prospective studies that controlled for specific type of medication, dose, and duration of antipsychotic treatment.
Furthermore, we found that a smoking cessation group therapy program for schizophrenic smokers that emphasized motivational enhancement, relapse prevention training, social skills training, and psychoeducation (2, 18) did not result in significantly different smoking cessation outcomes compared with a community-oriented smoking cessation program, when both programs were used in combination with the nicotine transdermal patch (16–18). The small size of our study group may have contributed to our inability to find significant differences in outcomes between these psychotherapy interventions. However, subjects in the specialized group therapy progam had significantly better rates of continuous smoking abstinence in the last 4 weeks of the trial than did those in the American Lung Association group (χ2=3.67, df=1, p=0.06). This difference is of clinical interest, since the cost of establishing and enrolling patients in community American Lung Association programs is high, and many schizophrenic patients do not tolerate the large amount of information and rigid format typically encountered in American Lung Association programs and other community-based smoking cessation programs (2). Both the treatment endpoint smoking cessation rate (about 35%) and abstinence rates at 6-month follow-up (about 20%–30%) in this study are lower than endpoint rates reported for nonpsychiatric smokers who attempt smoking cessation by using the nicotine transdermal patch (about 45%–70%) (36, 42). Thus, the development of more efficacious smoking cessation strategies for smokers with schizophrenic illness is of great importance. Several factors may predispose this population to heavy smoking. For example, cigarette smoking may reduce negative symptoms and cognitive deficits, attenuate stress-responsive symptom exacerbation, and ameliorate sensory processing abnormalities. Given these factors, a better understanding of the comorbidity of schizophrenic illness and nicotine dependence may lead to improved treatments for both disorders (2, 43, 44).
The differential effects of atypical versus typical agents in enhancing smoking cessation rates may also be explained through the reduction of antipsychotic-induced akathisia associated with atypical agents (45). Patients who take atypical agents generally have less akathisia, and since administration of nicotine has been shown to reduce neuroleptic-induced akathisia (46), atypical agents may thus have produced a secondary decrease in cigarette smoking by reduction of akathisia in schizophrenic smokers. We did not directly assess akathisia in this study, but we did find that a higher amount of adjunctive anticholinergic medication (which reduces akathisia) was used for subjects who received typical antipsychotics than for those who received atypical antipsychotics. This finding is consistent with the idea that extrapyramidal symptoms and akathisia were more common in the group who received typical antipsychotics. Future studies of cigarette smoking, atypical agents, and akathisia may help clarify these relationships.
At baseline in this study, subjects with schizoaffective disorder were significantly more likely to receive atypical antipsychotics than those with a diagnosis of schizophrenia. However, there were no differences in depressive symptoms at baseline or during the trial between those who received the two classes of antipsychotics (Table 2). Furthermore, subjects with schizoaffective disorder did not differ from those with schizophrenia on any smoking abstinence measure, either in comparisons of the diagnostic groups or in comparisons of the subgroups who received atypical or typical antispsychotics. Thus, it is unlikely that baseline differences between the groups receiving atypical and typical agents explain the group differences in smoking cessation outcomes that we observed. Furthermore, given that we did multiple comparisons involving several baseline group characteristics and study outcomes, some differences would be expected to occur by chance.
We found no changes in psychiatric symptoms as a function of group therapy, antipsychotic medication, or smoking status during the course of the nicotine transdermal patch trial. These findings are consistent with those of other studies that have shown no effects of smoking abstinence on schizophrenic symptoms with nicotine transdermal patch treatment (10, 47). Thus, although no differences in smoking abstinence outcomes were observed between the group psychotherapies tested in this preliminary trial with the nicotine transdermal patch, important differences emerged as a function of antipsychotic medication status. Further studies with other promising medications (e.g., sustained-release bupropion, nicotine inhaler) or psychosocial treatments (e.g., contingency management) (28) for smoking cessation in schizophrenic smokers are warranted. A better understanding of the factors that lead to successful smoking cessation outcomes in smokers with schizophrenia and other psychiatric disorders may lead to improved treatments for this subset of smokers, whose smoking habits are among the most refractory to conventional smoking cessation interventions.
 |
 |
Presented in part at the 38th annual meeting of the American College of Neuropsychopharmacology, Acapulco, Mexico, Dec. 12–16, 1999. Received March 6, 2000; revision received July 7, 2000; accepted July 10, 2000. From the Division of Substance Abuse, Department of Psychiatry, Yale University School of Medicine; the Dual Diagnosis & Smoking Research Program, Connecticut Mental Health Center; and the Division of Addiction Psychiatry, Department of Psychiatry, University of Medicine and Dentistry of New Jersey, Robert Wood Johnson Medical School, Piscataway.Address reprint requests to Dr. George, Division of Substance Abuse, Department of Psychiatry, Yale University School of Medicine, Connecticut Mental Health Center, 34 Park St., Rm. S-109, New Haven, CT 06519; [email protected] (e-mail). Supported in part by grants PDA-09250, PDA-09241, PDA-84733, and KDA-00167 from the National Institute on Drug Abuse; a VISN 1 Mental Illness Research Education and Clinical Center grant from the U.S. Department of Veteran Affairs; and a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression to Dr. George. The authors thank Jennifer C. Vessicchio, M.S.W., Angelo Termine, B.S., Stephen A. Wyatt, D.O., Connie Nickou, Psy.D., Christina Hernandez, and Kishorchandra R. Gonsai, M.D., for their assistance with this study, and Ross J. Baldessarini, M.D., Stephanie S. O’Malley, Ph.D., and Gregory W. Dalack, M.D., for their helpful comments on the manuscript.
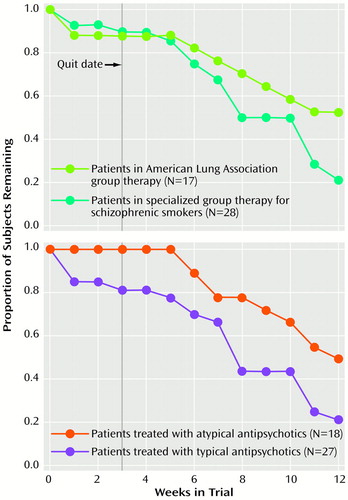
Figure 1. Treatment Retention in a 12-Week Smoking Cessation Trial for Schizophrenic Patients, by Group Therapy Program and by Antipsychotic Medication Classa
aDuring the first 10 weeks of the trial, patients participated in either a therapy group based on the American Lung Association’s smoking cessation program or a specialized therapy group for schizophrenic smokers. The smoking “quit date” occurred during week 3 of both group therapy programs. All patients received the nicotine transdermal patch during weeks 3–12. Throughout the 12-week trial, patients received the atypical or typical antipsychotic medication they had been taking before the trial.
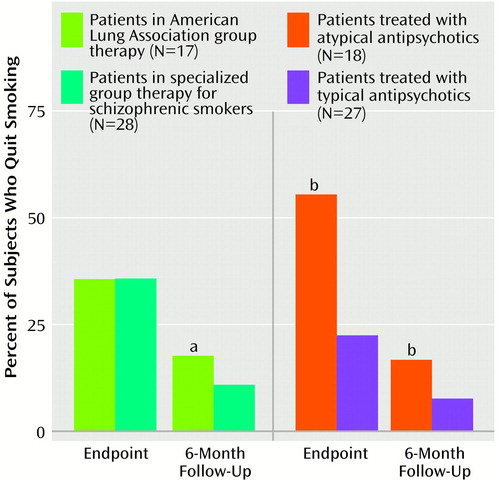
Figure 2. Smoking Abstinence Rates at the Endpoint of a 12-Week Smoking Cessation Trial and at 6-Month Follow-Up for Schizophrenic Patients, by Group Therapy Program and by Antipsychotic Medication Class
aSignificant difference between patients in the two group therapy programs (chi-square analysis, p<0.03).
bSignificant difference between patients treated with atypical anti-psychotics and those treated with typical antipsychotics (chi-square analysis, p<0.02).
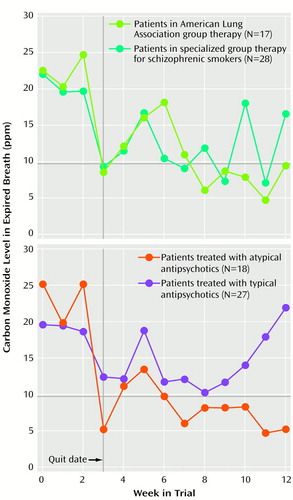
Figure 3. Subjects’ Expired Breath Carbon Monoxide Levels in a 12-Week Smoking Cessation Trial for Schizophrenic Patients, by Group Therapy Program and by Atypical or Typical Antipsychotic Medication Classa
aDuring the first 10 weeks of the trial, patients participated in either a therapy group based on the American Lung Association’s smoking cessation program or a specialized therapy group for schizophrenic smokers. The smoking “quit date” occurred during week 3 of both group therapy programs. All patients received the nicotine transdermal patch during weeks 3–12. Throughout the 12-week trial, patients received the atypical or typical antipsychotic medication they had been taking before the trial.
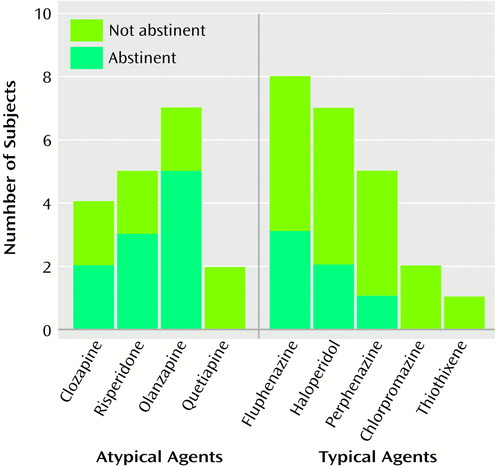
Figure 4. Smoking Abstinence Rates at the Endpoint of a 12-Week Smoking Cessation Trial for Schizophrenic Patients, by Specific Atypical or Typical Antipsychotic Medication
1. Consensus Statement on Evaluation of Outcomes for Pharmacotherapy for Substance Abuse/Dependence. Washington, DC, National Institute on Drug Abuse/College on Problems of Drug Dependence, April 1999. http://views.vcu.edu/cpdd/reports/nida_cpdd_report.pdfGoogle Scholar
2. Ziedonis DM, George TP: Schizophrenia and nicotine use: report of a pilot smoking cessation program and review of neurobiological and clinical issues. Schizophr Bull 1997; 23:247–254Crossref, Medline, Google Scholar
3. Dalack GW, Healy DJ, Meador-Woodruff JH: Nicotine dependence in schizophrenia: clinical phenomena and laboratory findings. Am J Psychiatry 1998; 155:1490–1501Google Scholar
4. Kelly C, McCreadie RG: Smoking habits, current symptoms, and premorbid characteristics of schizophrenic patients in Nithsdale, Scotland. Am J Psychiatry 1999; 156:1751–1757Google Scholar
5. Addington J, el Guebaly N, Addington D, Hodgins D: Readiness to stop smoking in schizophrenia. Can J Psychiatry 1997; 42:49–52Crossref, Medline, Google Scholar
6. Ziedonis DM, Trudeau K: Motivation to quit using substances among individuals with schizophrenia: implications for a motivation-based treatment model. Schizophr Bull 1997; 23:229–238Crossref, Medline, Google Scholar
7. Prochaska JO, DiClemente CC: Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol 1983; 51:390–395Crossref, Medline, Google Scholar
8. Allebeck P: Schizophrenia: a life-shortening disease. Schizophr Bull 1989; 15:81–89Crossref, Medline, Google Scholar
9. Mortensen PB: The incidence of cancer in schizophrenic patients. J Epidemiol Community Health 1990; 43:43–47Crossref, Google Scholar
10. Addington J, el Guebaly N, Campbell W, Hodgins DC, Addington D: Smoking cessation treatment for patients with schizophrenia. Am J Psychiatry 1998; 155:974–976Link, Google Scholar
11. McEvoy J, Freudenreich O, McGee M, VanderZwaag C, Levin E, Rose J: Clozapine decreases smoking in patients with chronic schizophrenia. Biol Psychiatry 1995; 37:550–552Crossref, Medline, Google Scholar
12. George TP, Sernyak MJ, Ziedonis DM, Woods SW: Effects of clozapine on smoking in chronic schizophrenia outpatients. J Clin Psychiatry 1995; 56:344–346Medline, Google Scholar
13. McEvoy JP, Freudenreich O, Wilson WH: Smoking and therapeutic response to clozapine in patients with schizophrenia. Biol Psychiatry 1999; 46:125–129Crossref, Medline, Google Scholar
14. McEvoy J, Freudenreich O, Levin E, Rose J: Haloperidol increases smoking in patients with schizophrenia. Psychopharmacology (Berl) 1995; 119:124–126Crossref, Medline, Google Scholar
15. Evins AE, Tisdale T: Bupropion and smoking cessation (letter). Am J Psychiatry 1999; 156:798–799Medline, Google Scholar
16. George TP, Pepper WT, Satterburg CA, Vessicchio JC, Jackson TD, Madonick SH, Kosten TR: Pharmacotherapy for smoking cessation in schizophrenia: biological correlates. Abstracts of the American College of Neuropsychopharmacology 1999; 38:119Google Scholar
17. Evins AE, Mays VK, Rigotti NA, Tisdale T, Daigle A, Goff DC: Reduction in tobacco use in schizophrenia with bupropion SR and cognitive behavioral therapy, in Abstracts of the 6th Annual Meeting of the Society for Research on Nicotine and Tobacco. Middleton, Wis, SRNT, 2000Google Scholar
18. Rosen-Chase C, Dyson V: Treatment of nicotine dependence in the chronic mentally ill. J Subst Abuse Treat 1999; 16:315–320Crossref, Medline, Google Scholar
19. Addington J: Group treatment for smoking cessation among persons with schizophrenia. Psychiatr Serv 1998; 49:925–928Link, Google Scholar
20. Schimtz JM, Rhoades H, Grabowski J: Contingent reinforcement for reduced carbon monoxide levels in methadone maintenance patients. Addict Behav 1995; 20:171–179Crossref, Medline, Google Scholar
21. Shoptaw S, Jarvik ME, Ling W, Rawson RA: Contingency management for tobacco smoking in methadone-maintained opiate addicts. Addict Behav 1996; 21:409–412Crossref, Medline, Google Scholar
22. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
23. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571Crossref, Medline, Google Scholar
24. Shiffman SM, Jarvik ME: Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology (Berl) 1976; 50:35–39Crossref, Medline, Google Scholar
25. Andersen K, Balldin J, Gottfries CG, Granerus AK, Modigh K, Svennerholm L, Wallin A: A double-blind evaluation of electroconvulsive therapy in Parkinson’s disease with “on-off” phenomena. Acta Neurol Scan 1987; 76:191–199Crossref, Medline, Google Scholar
26. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 534–537Google Scholar
27. Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO: The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 1991; 86:1119–1127Google Scholar
28. Roll JM, Higgins ST, Steingard S, McGinley M: Use of monetary reinforcement to reduce the cigarette smoking of persons with schizophrenia: a feasibility study. Exp Clin Psychopharmacol 1998; 6:157–161Crossref, Medline, Google Scholar
29. McGovern PG, Lando HA: An assessment of nicotine gum as an adjunct to freedom from smoking cessation clinics. Addict Behav 1992; 17:137–147Crossref, Medline, Google Scholar
30. Martin JE, Calfas KJ, Patten CA, Polarek M, Hofstetter CR, Noto J, Beach D: Prospective evaluation of three smoking interventions in 205 recovering alcoholics: one-year results of Project SCRAP-Tobacco. J Consult Clin Psychol 1997; 65:190–194Crossref, Medline, Google Scholar
31. Miller WR, Rollnick S: Motivational Interviewing: Preparing People to Change Addictive Behavior. New York, Guilford Press, 1991Google Scholar
32. Bland JM, Altman DG: Survival probabilities (the Kaplan-Meier method). Br Med J 1998; 317:1572Crossref, Medline, Google Scholar
33. Bryk AS, Raudenbush SW: Application of hierarchical linear models to assessing change. Psychopharmacol Bull 1987; 101:147–158Google Scholar
34. Gibbons RD, Hedeker D, Elkin I, Waternaux C, Kraemer HC, Greenhouse JB, Shea MT, Imber SD, Sotsky SM, Watkins JT: Some conceptual and statistical issues in analyses of longitudinal psychiatric data. Arch Gen Psychiatry 1993; 50:739–750Crossref, Medline, Google Scholar
35. Schatzberg AF, Cole JO, DeBattista C: Manual of Clinical Psychopharmacology, 3rd ed. Washington, DC, American Psychiatric Press, 1997Google Scholar
36. Balfour DJK, Fagerstrom KO: Pharmacology of nicotine and its therapeutic use in smoking cessation and neurodegenerative disorders. Pharmacol Ther 1996; 72:51–81Crossref, Medline, Google Scholar
37. Albanese MJ, Khantzian EJ, Murphy SL, Green AI: Decreased substance use in chronically psychotic patients treated with clozapine (letter). Am J Psychiatry 1994; 151:780–781Medline, Google Scholar
38. Buckley P, Thompson P, Way L, Meltzer HY: Substance abuse among patients with treatment-resistant schizophrenia: characteristics and implications for clozapine therapy. Am J Psychiatry 1994; 151:385–389Link, Google Scholar
39. Swerdlow NR, Caine SB, Braff DL, Geyer MA: The neural substrates of sensorimotor gating of the startle reflex: a review of recent findings and their implications. J Psychopharmacol 1992; 6:176–190Crossref, Medline, Google Scholar
40. Nagamoto HT, Adler LE, Waldo MC, Griffith JM, McRae KA, Freedman R: Gating of auditory P50 in schizophrenics: unique effects of clozapine. Biol Psychiatry 1996; 40:181–188Crossref, Medline, Google Scholar
41. Kumari V, Checkly SA, Gray JA: Effect of cigarette smoking on prepulse inhibition of the acoustic startle reflex in healthy male smokers. Psychopharmacology (Berl) 1996; 128:54–60Crossref, Medline, Google Scholar
42. Ziedonis DM, Wyatt SA, George TP: Current issues in nicotine dependence and treatment, in New Treatments for Chemical Addictions. Edited by McCance-Katz EF, Kosten TR. Washington, DC, American Psychiatric Press, 1998, pp 1–34Google Scholar
43. George TP, Verrico CD, Roth RH: Effects of repeated nicotine pretreatment on mesoprefrontal dopaminergic and behavioral responses to acute footshock stress. Brain Res 1998; 801:36–49Crossref, Medline, Google Scholar
44. George TP, Verrico CD, Xu L, Roth RH: Effects of repeated nicotine administration and footshock stress on rat mesoprefrontal dopamine systems: evidence for opioid mechanisms. Neuropsychopharmacology 2000; 23:79–88Crossref, Medline, Google Scholar
45. Gerlach J, Larsen EB: Subjective experience and mental side-effects of antipsychotic treatment. Acta Psychiatr Scand Suppl 1999; 395:113–117Crossref, Medline, Google Scholar
46. Anfang MK, Pope HG Jr: Treatment of neuroleptic-induced akathisia with nicotine patches. Psychopharmacology (Berl) 1997; 134:153–156Crossref, Medline, Google Scholar
47. Dalack GW, Becks L, Hill E, Pomerleau OF, Meador-Woodruff JH: Nicotine withdrawal and psychiatric symptoms in cigarette smokers with schizophrenia. Neuropsychopharmacology 1999; 21:195–202Crossref, Medline, Google Scholar



