The Potential for Viral Gene Therapy in Psychiatry
Research has implicated specific regions in the human brain that are associated with pathological manifestations of distinct psychiatric disorders. Often and increasingly, disease-specific molecular alterations have been implicated in these regions. Through advances in research, neuroscience is approaching the goal of identifying molecular alterations specific to depression, psychosis, and drug abuse. Similar progress in Parkinson’s disease, epilepsy, and dementia research has been attained.
In the images presented, an animal model of drug abuse is illustrated. These images illustrate the anatomical and molecular changes that correlate with the behavioral manifestations of drug abuse. Specifically, chronic but not acute use of cocaine or amphetamines increases the number of dendritic spines (panel A) on medium spiny neurons (panel B) in the nucleus accumbens (NAc). This structural change in the medium spiny neuron correlates with cocaine-seeking behavior and increased risk of relapse in animal models of addiction. Moreover, our research has identified a transcription factor, nuclear factor kappa B (NFkB), which mediates this structural change. Importantly, inhibition of NFkB signaling through the use of viral gene therapy techniques with an inhibitor of kappa B kinase (panel B) blocks NFkB signaling and the rewarding effects of cocaine in animals. In addition, antagonizing NFkB also blocks the ability of prior cocaine exposure to increase an animal’s preference for cocaine. If these results from animal studies translate to human use of cocaine, one might predict that NFkB inhibition could block human cocaine abuse. In previous decades, using this finding to develop a treatment for cocaine abuse would have involved pharmacological approaches that established an antagonist of NFkB.
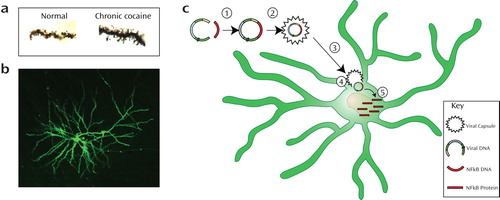
Figure 1. As accessed by Golgi-Cox staining, medium spiny neurons (MSNs) in the nucleus accumbens increase the number of dendritic spines following chronic cocaine abuse. Panel A shows cocaine-induced increases in dendritic spines after chronic cocaine abuse. Using neurosurgical techniques, a viral vector is injected into the nucleus accumbens to insert modified DNA in the MSNs, which can then express a specialized protein (panel B). Panel C illustrates a cartoon schematic. Viral DNA is fused with NFkB DNA and packaged inside a viral capsule. This capsule can then introduce the DNA within the soma of a medium spiny neuron to produce the NFkB protein and regulate the activity of this signaling pathway. (Image courtesy of Dr. Russo.)
Recent molecular methodologies have suggested another anatomically focused experimental therapeutic strategy for the future, which involves the use of viral vectors. In this therapeutic strategy, genetic material for NFkB inhibition would be introduced into the pathological region in the brain using a viral vector. The viral material would facilitate the passage of the NFkB-related DNA into the cells. Once inside the cell, the exogenous DNA would produce specialized proteins that alter cell function. This type of method is being used in animal studies, as described previously, to demonstrate that NFkB antagonizes cocaine sensitization. Although this process is only currently being developed for human brain diseases, it is a standard procedure for effecting genetic manipulations in rodents. The schematic in panel C illustrates this process as follows: 1) the gene (DNA) that encodes for a particular protein of interest is fused into the viral DNA; 2) the fused DNA is then packaged inside the viral capsule; 3) the DNA viral package (viral vector) is introduced into the target brain region using neurosurgical techniques; 4) the viral vector containing the genetically engineered DNA can then enter a cell and become active in the soma of the “infected” cells and 5) become regularly transcribed to produce the cDNA-coded protein. This procedure allows an internal cellular process to produce a target protein product locally, which has therapeutic actions.
Viral vector treatments are already being clinically tested in Parkinson’s disease and epilepsy patients. Psychiatric brain diseases, such as cocaine addiction and depression, are more distant, but not unrealistic, possibilities for viral vector therapeutics.



