Increased Amygdala and Insula Activation During Emotion Processing in Anxiety-Prone Subjects
Abstract
Objective: Increased amygdala reactivity during processing of certain types of emotional stimuli (e.g., fear, anger) has been observed in patients with anxiety disorders such as social phobia and posttraumatic stress disorder (PTSD). It is uncertain whether this heightened amygdala reactivity is specific to treatment-seeking patients with anxiety disorders or is a general feature of individuals with increased anxiety-related temperamental traits. Method: Thirty-two physically healthy subjects 18–21 years old were recruited from a large pool of college students. Of these, 16 were chosen on the basis of scoring in the upper-15th percentile on a measure of trait anxiety (anxiety-prone group), and 16 were chosen on the basis of scoring in the normative range (40th–60th percentile). Subjects participated in functional magnetic resonance imaging (fMRI) during an emotion face assessment task that has been shown to reliably engage amygdala and associated limbic structures. Results: Anxiety-prone subjects had significantly greater bilateral amygdala and insula activation to emotional faces than did the anxiety-normative comparison subjects. Higher scores on several measures assessing anxiety proneness (e.g., neuroticism, trait anxiety, and anxiety sensitivity) were associated with greater activation of the amygdala (predominantly left-sided) and the anterior insula (bilateral). Conclusions: Increased amygdala and insula reactivity to certain types of emotional processing is seen in young adults with increased anxiety-related temperamental traits. Therefore, this brain emotion-processing profile may be a functional endophenotype for proneness to (certain kinds of) anxiety disorders.
Anxiety disorders are the most prevalent category of mental illness in the United States (1) and other countries (2) and are often associated with marked decrements in functioning and quality of life (3 – 5) . In addition to these direct deleterious effects, anxiety disorders—which typically have their onset early in life (6) —increase the risk for the subsequent onset of depressive disorders (7 , 8) .
Given the importance of anxiety disorders, considerable effort is being directed toward better understanding their biological underpinnings. The amygdala plays a critical role in normal fear conditioning (9 – 12) and is increasingly being implicated in the pathophysiology of anxiety disorders (13 , 14) . For example, impaired ability to recognize fear from facial expressions as well as a lack of fearfulness in social contexts and the failure to acquire conditioned fear responses (15 , 16) are observed after amygdala damage in humans. Further evidence for a critical role of the amygdala in the response to fear stems from observation of its activation to emotional (usually fearful or angry) human faces (possibly to the eyes themselves) (17) in numerous positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) studies (13 , 18 – 20) .
Exaggerated amygdala activation to emotional human faces has been noted in several of the anxiety disorders, namely social anxiety disorder (21 – 24) , and posttraumatic stress disorder (PTSD) (25 , 26) and, less definitively, in panic disorder and generalized anxiety disorder (27) . In contrast, amygdala hyperactivity has not been observed in either specific phobia (28) or obsessive-compulsive disorder (29) . These differential task effects across anxiety disorders serve as a reminder that the neural circuitry of all anxiety disorders is not uniform.
Furthermore, Schwartz et al. (30) recently observed that exaggerated amygdala responses to novel emotional faces occur in adults identified in childhood as having the anxiety-related temperamental risk factor “behavioral inhibition,” even in the absence of a current DSM–IV anxiety disorder diagnosis, which prompted the authors to note the following:
[D]iscovery of a difference in brain activity between subjects with a psychiatric diagnosis and a control group should not always be regarded as a specific marker of the disorder. The difference may reflect instead a temperamental risk factor, or diathesis, for the diagnostic category under study. (p. 1953)
One approach to testing the aforementioned hypothesis would involve studying amygdala activation to emotional processing in nonclinical subjects (i.e., those who have not sought treatment for anxiety disorders), yet have high levels of anxiety-related traits that would be considered to render them “anxiety prone.” This approach may offer the advantage of providing insights into processes that underlie anxiety in both normative and pathological conditions (31) . Temperamental risk factors that would be prime candidates for such investigation would include neuroticism, which is probably a nonspecific risk factor for anxiety and depressive disorders (32 – 34) , and anxiety sensitivity (the fear of anxiety-related sensations) (35) , which may be a more specific risk factor for certain anxiety disorders (e.g., panic disorder) (36 – 38) .
Investigating subjects with anxiety proneness provides a unique opportunity to examine the neural systems that are important for mediating increased levels of anxiety and enables one to understand the processes that may be responsible for the development of anxiety disorders. It would be inappropriate, however, to confine such investigation to the amygdala. In addition to the amygdala, a network of structures that includes the insula, anterior cingulate gyrus, and medial prefrontal cortex is important to identify the emotional significance of a stimulus, generate an affective response, and regulate the affective state (39 , 40) . The insula has afferent and efferent connections to the medial and orbitofrontal cortices, anterior cingulate, and amygdala (41) . Although insula activation has been frequently associated with disgust (42) , there is increasing evidence of a broader role for this brain structure in emotion processing (43) . Insula activation is also thought to be involved in differential positive versus negative emotion processing (44) and in the making of judgments about emotions based on facial expression (45) . There is thus ample reason to consider the possibility that insula function may have been relatively neglected (i.e., compared with amygdala function) in human studies of anxiety-related psychopathology and to include the insula within our sphere of inquiry in this study.
The aim of this study was to use blood-oxygenation-level-dependent fMRI in combination with performance of an emotion-processing task known to engage limbic circuitry (46 – 48) to test the hypothesis that relative to subjects with normative levels of anxiety proneness, high anxiety-prone individuals show exaggerated activation in the amygdala and insula (but not in the fusiform gyrus, a region critical for the coding of facial stimuli) (49 – 51) during an emotional face paradigm. In light of recent observations that medial prefrontal cortex activity may be reduced in patients with posttraumatic stress disorder (PTSD) in concert with amygdala hyperreactivity, perhaps reflecting inadequate top-down regulation of the amygdala by the medial prefrontal cortex in patients with anxiety disorders (26 , 52) , we also focused on task-related activation in the medial prefrontal cortex and its relationship with amygdala and insula activity in both the anxiety-prone group and the anxiety-normative group. These analyses were expected to shed additional light on the role of the amygdala and insula as key components in the neural circuits that mediate anxiety-related symptoms.
Method
Participants
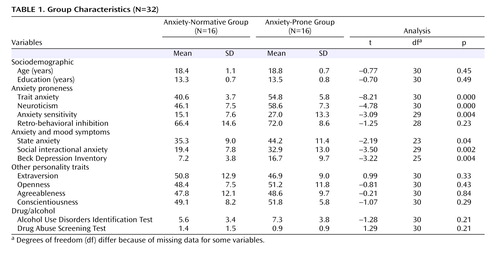
This study was approved by the institutional review boards of the University of California, San Diego and San Diego State University. All subjects provided written informed consent to participate. Initially, approximately 3,000 undergraduate students from San Diego State University participated in screening using the Spielberger State-Trait Anxiety Inventory (53) . Subsequently, subjects who scored high in trait anxiety (in the upper-15th percentile of the distribution) and subjects who had normative levels of trait anxiety (from the 40th–60th percentile of the distribution) were selected for further screening. Of these, approximately one in three expressed a willingness to participate in an fMRI study; approximately one in two of those willing to participate in an fMRI study could be contacted for further assessment; and approximately one in two of those who could be contacted proved eligible. Of those who proved eligible, 32 subjects were able to be studied during our available fMRI time slots: 14 women and two men with normal-trait anxiety scores (anxiety normative) and 12 women and four men with high-trait anxiety scores (anxiety prone [continuity corrected chi square=0.251, df=1, p=0.65]).
All subjects underwent a Structured Clinical Interview for DSM-IV (SCID-I) (54) , which was modified to enable us to document the presence of subthreshold (i.e., did not fulfill full DSM–IV criteria because of insufficient number of symptoms and/or below diagnostic threshold for distress and/or interference) anxiety and mood disorders. Anxiety-prone subjects could have a DSM–IV diagnosis (full or subthreshold), but were not currently seeking or had ever sought treatment for their anxiety symptoms in the past. In the anxiety-prone group, seven subjects had no DSM–IV diagnosis (six of these subjects had subthreshold generalized anxiety disorder and/or social anxiety disorder); five subjects had generalized anxiety disorder only; three subjects had generalized anxiety disorder with social anxiety disorder; and one subject had generalized anxiety disorder, social anxiety disorder, panic disorder, and obsessive-compulsive disorder. Anxiety-normative subjects were those who were determined to have no DSM–IV disorders, even at the subthreshold level. None of the subjects had taken any psychotropic medications in the prior 12 months. Subjects habitually consumed less than 400 mg of caffeine daily. All subjects were trained to perform the emotional face task prior to testing during fMRI scanning. Subjects were paid to participate in the fMRI study.
Measures
Subjects completed the NEO Five Factor Inventory (55) , a widely used 60-item self-report measure of personality, grouped into the following five major domains: neuroticism (N), extroversion (E), openness to experience (O), conscientiousness (C), and agreeableness (A). Subjects also completed the Anxiety Sensitivity Index, a 16-item self-report measure of the fear of anxiety-related sensations (35) , and the Retrospective Self-Report of Behavioral Inhibition, a 30-item measure in which items were chosen to represent a broad range of behaviors associated with behavioral inhibition (56) . The Alcohol Use Disorders Identification Test (57) and the Drug Abuse Screening Test (58) were also included.
Task
During fMRI, each subject was tested on a slightly modified (47) version of the Emotion Face Assessment Task (46 , 48) . During each 5-second trial, a subject was presented with a target face (on the top of the computer screen) and two probe faces (on the bottom of the screen) and was instructed to match the probe with the same emotional expression to the target by pressing the left or right key on a button box. A block consisted of six consecutive trials where the target face is angry, fearful, or happy. During the sensorimotor control task, subjects were presented with 5-second trials of either wide or tall ovals or circles in an analogous configuration and instructed to match the shape of the probe to the target. Each block of faces and of the sensorimotor control task was presented three times in a pseudorandomized order. A fixation cross lasting 8 seconds was interspersed between each block presented at the beginning and end of the task (resulting in 14 fixation periods). For each trial, response accuracy and reaction time data were obtained. There were 18 trials (three blocks of six trials) for each face set as well as for shapes. The whole task lasted 512 seconds (matching the scan length).
Image Acquisition
During the task, one blood-oxygenation-level-dependent fMRI run was collected for each subject using a 1.5-Tesla Siemens (Erlangen, Germany) scanner (T2-weighted echo planar imaging, TR=2000 msec, echo time=40 msec, 64×64 matrix, 20 4-mm axial slices, 256 repetitions). During the same experimental session, a T1-weighted image (MPRAGE, TR=11.4 msec, echo time=4.4 msec, flip angle=10°, field of view=256×256, 1 mm 3 voxels) was obtained for anatomical reference. For preprocessing, voxel time series were interpolated to correct for nonsimultaneous slice acquisition within each volume and corrected for three-dimensional motion.
Image Processing and Analysis
All structural and functional image processing was done with the Analysis of Functional Neuroimages software package (59) . Echo planar image intensity images were coregistered to the 128th image using a three-dimensional-coregistration algorithm. The time series of the alignments in the x, y, z and roll, pitch, yaw directions was used to obtain motion regressors for each subject. Because small motion corrections are similar in angle (e.g., roll) and displacement (e.g., x), we used only three motion parameters (roll, pitch, yaw) as nuisance regressors to account for motion artifacts. The four orthogonal regressors of interest were 1) happy, 2) angry, 3) fearful, and 4) circle/oval (i.e., shape) sensorimotor condition. These regressors were convolved with a modified gamma variate function to account for the delay and the dispersion brain response of the blood-oxygenation-level-dependent fMRI signal because of hemodynamics (60 , 61) . Additional regressors were used to model residual motion in the roll, pitch, and yaw directions as well as baseline and linear tendencies. The Analysis of Functional Neuroimages program three-dimensional deconvolve was used to calculate the estimated voxel-wise response amplitude. A Gaussian filter with full width at half maximum 4 mm was applied to the voxel-wise percent signal change data to account for individual variations in the anatomical landmarks.
Data for each subject were normalized to Talairach coordinates. Whole brain analyses were followed by a priori analysis of regions of interest using masks (defined by the Talairach demon atlas) (62) in the bilateral amygdala, insula, ventromedial prefrontal cortex (ventromedial prefrontal cortex; consisting of anterior cingulate, subgenual cingulate, and medial frontal gyrus, corresponding to Brodmann’s areas 24, 25, and 32) and primary visual cortex. Based on these areas of interests, it was determined via simulations that a voxel-wise a priori probability of 0.05 would result in a corrected cluster-wise activation probability of 0.05 if a minimum volume of 128 μl and two connected voxels (in the amygdala, which is a very small structure) or 512 μl and eight connected voxels (in all other regions of interest) were considered. The areas of interest were superimposed on each individual’s voxel-wise percent signal change brain image. Only activations within the areas of interest, which also satisfied the volume and voxel connection criteria, were extracted and used for further analysis. The corrected voxel-wise probabilities are as follows: amygdala, p<0.01; insular cortex, p<0.00007; medial prefrontal cortex, p<0.0001; and visual cortex, p<0.00007. These corrected voxel probabilities are based on Monte Carlo simulations using Analysis of Functional Neuroimages program AlphaSim with the filtered data and the a priori defined regions of interest.
Statistical Analysis
All behavioral analyses were carried out with SPSS 11.01. A between-subjects multivariate analysis of variance (MANOVA) was used to analyze the behavioral measures and neural activation patterns. For the imaging analyses, the voxel-wise percent signal change data were entered into a mixed-model analysis of variance (ANOVA) with task contrast (face type-shape comparison condition) and group (anxiety-prone or anxiety-normative condition) as fixed factors and subjects as a random factor. We conducted correlational analyses to examine the relationship between self-rating scales of anxiety proneness and other traits and activation (as a percentage change from the baseline, shape-only condition) in the amygdala and insula during viewing of angry, fearful, and happy faces. We also conducted correlational analyses of the task-related activation in the ventromedial prefrontal cortex with that in the amygdala and insula; these analyses were conducted for anxiety-prone and anxiety-normative groups separately and for both groups combined.
Results
Group Characteristics
The two groups were significantly different on several indices of anxiety proneness ( Table 1 ), including trait anxiety (which was the group selection criterion), neuroticism, and anxiety sensitivity, but not on retrospective behavioral inhibition. They also differed significantly on several measures of anxious and depressive symptoms. However, they were not significantly different in terms of age or years of education, nor did they differ on other measures of personality or drug and alcohol use.
Group Differences in Response Latency or Accuracy
There were no significant group differences in response latency or accuracy ( Figure 1 ).

Group Differences in Functional MRI Blood-Oxygenation-Level-Dependent Activity
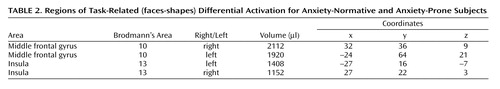
Whole brain analysis revealed significant task-related differences between anxiety-prone and anxiety-normative subjects in several regions ( Table 2 ). Among these regions was the bilateral anterior insular cortex ( Figure 2 ), with anxiety-prone subjects having significantly greater activation for contrasts between emotional faces and shapes; in fact, anxiety-prone subjects tended to activate, while anxiety-normative subjects showed deactivation for this contrast (whereas there was no significant main effect of task in the insular cortex). Whole brain analysis revealed task-specific activation in the amygdala in both groups, but significant differences between groups were evident only in the amygdala region-of-interest analyses, which detected significantly greater bilateral (although more pronounced on the left side) amygdala activation in anxiety-prone subjects compared with anxiety-normative subjects ( Figure 3 ). Different face types (angry, fear, or happy) did not differentially activate insular cortex or amygdala, nor was there a significant interaction between-group and face type in these structures. There were no significant differences in fusiform activation between anxiety-prone and anxiety-normative subjects.

a Left: Extent of insula activation for each type of emotional face versus shape contrast. Right: Activation area for emotional faces versus shapes showing significantly increased activation in bilateral insula for anxiety-prone subjects. Talairach coordinates for left insula center of mass (x, y, z): –27, 16, –7 (volume 1408 μl); right insula: 27, 22, 3 (volume 1152 μl).

a Left: Extent of bilateral amygdala activation for each type of emotional face versus shape contrast. Right: Region of interest analysis of emotional faces versus shapes showing significantly increased activation in bilateral amygdala for anxiety-prone subjects. Talairach coordinates for left amygdala activation center of mass (x, y, z): –22, –9, –10 (volume 128 μl); right amygdala: 23, –6, –13 (volume 512 μl).
Relationships Between Measures of Anxiety Proneness and Limbic Activation
We examined correlations between the measures of anxiety proneness and the extent of task-related activation in the amygdala and insula (and, for comparison purposes, with the fusiform gyrus, where we did not expect to see significant correlations). For both structures, mean activation (emotional faces, shapes, percentage) was extracted from regions of activation (coordinates shown in Figure 2 and Figure 3 ); correlations with other measures of activation (e.g., angry faces, shapes, percentage yielded very similar results.
Insula Activation
Greater task-related insula activation in the bilateral insular cortex was associated with higher levels on the measures of anxiety proneness that differentiated the groups (Spielberger State-Trait Anxiety Inventory, right insula: r=0.41, df=31, p=0.02; left insula: r=0.54, df=31, p=0.001; NEO Neuroticism, right insula: r=0.41, df=31, p=0.02; left insula: r=0.59, df=31, p<0.0005; Anxiety Sensitivity Index [ Figure 4 ], right insula: r=0.55, df=30, p=0.001; left insula: r=0.54, df=30, p=0.002).

Amygdala Activation
Greater bilateral task-related amygdala activation was associated with higher Spielberger State-Trait Anxiety Inventory (right amygdala: r=0.42, df=31, p=0.018; left amygdala: r=0.45, df=31, p=0.010), unilaterally with NEO Neuroticism (right amygdala: r=–0.030, df=31, p=0.87; left amygdala: r=0.391, df=31, p=0.027), but not with Anxiety Sensitivity Index (right amygdala: r=0.095, df=30, p=0.61; left amygdala: r=0.274, df=30, p=0.14).
Fusiform Activation
There was no relationship between task-related fusiform gyrus activation and any of the measures of anxiety proneness.
Relationship With Activation in Ventromedial Prefrontal Cortex
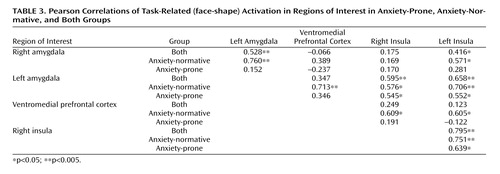
In order to determine whether task-related limbic and paralimbic activations in the amygdala and insular cortex were associated with top-down modulatory areas, we examined correlations between task-related activation in an anatomical region of interest in the ventromedial prefrontal cortex (Brodmann’s areas 24, 25, 32, including the anterior cingulate, subgenual cingulate, and medial frontal gyrus) and activation in the insula and amygdala ( Table 3 ). Most correlations were positive, and there were several ( Table 3 ) where the correlation with the ventromedial prefrontal cortex in anxiety-normative subjects was more than twice as strong as in anxiety-prone subjects. This difference in magnitude of correlations reached statistical significance (p=0.04) only for the correlation of the left insula with the ventromedial prefrontal cortex.
Discussion
Using an emotion-processing task previously demonstrated to engage limbic circuitry (46 , 47) , we found increased amygdala and insula activation in young adult anxiety-prone nonpatients relative to subjects with normative levels of anxiety proneness. We also found that the magnitude of activation in these limbic regions correlated moderately with several measures of anxiety proneness, such as anxiety sensitivity and neuroticism. Thus, although increased amygdala responsiveness has been seen in several groups of patients with anxiety (and depressive) disorders, our findings suggest that it extends to individuals with subthreshold symptoms and/or traits, such as neuroticism or anxiety sensitivity, that can be considered to characterize them as anxiety prone. This interpretation is consistent with observations from other research groups that reported amygdala activation to emotional (e.g., fearful) faces to be associated with individual variation in anxiety-related personality traits, such as threat sensitivity (63) or social anxiety (64) .
The increased amygdala activation to emotional face processing, which has been observed in many studies of patients with anxiety disorders (13) , sets the stage for our finding that this phenomenon extends to our nonclinical cohort of anxiety-prone persons and is, in fact, echoed in another recent report of persons described as “phobia-prone” (65) . The finding of increased insula activation in anxiety-prone subjects, however, may not have been as expected, based on the extant literature on anxiety disorders. It is difficult to know whether anxiety disorder studies have failed to find differential insular activation (with the exception of a study of specific phobia, where increased right insular activation was seen) (28) or whether this has not been adequately explored. However, in addition to the considerable preclinical literature that posits a role for the insula in the recognition of emotion in faces (45 , 66 , 67) and human functional neuroimaging work that demonstrates insular activation during the processing of salient emotional images (68) , human studies strongly suggest that the insula is instrumental in the detection and interpretation of certain internal bodily states (69 , 70) . The right insula, in particular, has been associated with the extent of “interoceptive awareness” of and discomfort with one’s own physiological (heart rate) response to emotionally valent pictures (71) . This construct of interoceptive awareness shares many features with anxiety sensitivity, which as we already noted is elevated in persons prone to anxiety disorders and is correlated with insula activity in our study. We hope that these observations will lead to more careful scrutiny of insular activity in future studies of psychopathology so that the role of this structure in emotion processing and its functional relationship to other elements of anxiety-related circuitry can be more fully elaborated (72) .
Given recent observations in patients with PTSD that there may be deficient reciprocal inhibitory control of amygdala activation by the ventromedial prefrontal cortex (26 , 52) , we evaluated relationships between task-related activation in the ventromedial prefrontal cortex with that in the insular cortex and amygdala. Contrary to expectation, we did not find negative correlations between the ventromedial prefrontal cortex activity and activity in the insula and/or amygdala. However, we did find that anxiety-normative subjects tended to have stronger correlations between the ventromedial prefrontal cortex and bilateral insula (and left-sided amygdala) than did anxiety-prone subjects, supporting the notion of increased functional “connectivity” in the anxiety-normative group. This increased amygdala ventromedial prefrontal cortex coupling, as indexed by increased correlated activity, may be consistent with emerging literature suggesting that increased integration of these functional connections reflects a more adaptive (e.g., less anxious) emotional phenotype (73 , 74) . We must emphasize, however, that statistical power to test for significant differences in the strength of correlations between groups was low and, hence, these results must be considered preliminary. We do intend to conduct more extensive functional connectivity analyses (75) in this data set to permit a more detailed exploration of cortical-limbic relationships; these data will be published separately.
This study has a number of other important limitations. Although the cohort size was sufficient to clearly demonstrate differences in the extent of amygdala and insula activation between the anxiety-normative and anxiety-prone groups, these region-specific comparisons were predicated on a priori hypotheses, and it is possible that other regional differences went undetected. Similarly, although we found significant correlations between amygdala and insula activation and various measures of anxiety proneness, it is possible that cryptic confounders (i.e., other individual differences that we did not think to measure) better explain these relationships. The data are cross-sectional, and some of the individuals identified as “anxiety prone” already showed evidence of having an anxiety disorder; thus, prospective data will be needed to definitively separate future risk from current symptoms. The predominance of women in this study—although reflecting the increased prevalence of anxiety disorders in women in the community—may mean that the findings cannot be generalized to men. Each of these limitations necessitates that our results be replicated. When such replications occur, it will be important to document characteristics of the functional neuroimaging task, such as test-retest reliability, and to include other tasks that engage the same (and disparate) emotion-processing circuits.
Findings from this study highlight some of the limits of our understanding of the relationship between anxiety-related psychopathology and brain circuitry and pose several questions that can only be answered with further research. It seems clear from these data that altered amygdala functioning is not disorder specific, nor is it necessarily indicative of psychopathology per se . Thus, attempts to cross-validate our current anxiety disorder diagnostic categories with evidence of functional alterations in specific circuits are unlikely to prove feasible. On the other hand, it may be possible to start with the observation of functional differences in amygdala and/or insula functioning in certain individuals and then determine what other characteristics they share (e.g., longitudinal course, treatment outcome). In other words, exaggerated amygdala and insula hyperactivity to certain types of emotional processing could tentatively be considered an endophenotype that may transcend our current diagnostic categories and serve as the cornerstone for further empirical nosological investigations.
In fact, there is already evidence amassing that the amygdala response to emotional face processing is moderated by genetic factors such as functional variation in the serotonin transporter, (46 , 48 , 65 , 73 , 74) raising the possibility that this particular endophenotype may well lend itself to further biological characterization. The question, then, remains, “What is the nature of this endophenotype?” Is it a prototype for anxiety proneness, for mood- and anxiety-related psychopathology more generally, or for some even broader construct, such as emotional resilience? In order to answer this question, functional neuroimaging will need to be conducted on an epidemiological scale, with close attention to issues of cohort frame, generalizability, confounders, and reliability. Only under such rigorous conditions will it be possible in the future to use neurobiological measures in general, and functional neuroimaging findings in particular, as the basis for a diagnostic system for anxiety- and mood-related psychopathology.
1. Kessler RC, Chiu WT, Demler O, Walters EE: Prevalence, severity, and comorbidity of 12-month DSM–IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62:617–627Google Scholar
2. Demyttenaere K, Bruffaerts R, Posada-Villa J, Gasquet I, Kovess V, Lepine JP, Angermeyer MC, Bernert S, de Girolamo G, Morosini P, Polidori G, Kikkawa T, Kawakami N, Ono Y, Takeshima T, Uda H, Karam EG, Fayyad JA, Karam AN, Mneimneh ZN, Medina-Mora ME, Borges G, Lara C, de Graaf R, Ormel J, Gureje O, Shen Y, Huang Y, Zhang M, Alonso J, Haro JM, Vilagut G, Bromet EJ, Gluzman S, Webb C, Kessler RC, Merikangas KR, Anthony JC, Von Korff MR, Wang PS, Brugha TS, Aguilar-Gaxiola S, Lee S, Heeringa S, Pennell BE, Zaslavsky AM, Ustun TB, Chatterji S: Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization world mental health surveys. JAMA 2004; 291:2581–2590Google Scholar
3. Mendlowicz MV, Stein MB: Quality of life in individuals with anxiety disorders. Am J Psychiatry 2000; 157:669–682Google Scholar
4. Alonso J, Angermeyer MC, Bernert S, Bruffaerts R, Brugha TS, Bryson H, Girolamo G, Graaf R, Demyttenaere K, Gasquet I, Haro JM, Katz SJ, Kessler RC, Kovess V, Lepine JP, Ormel J, Polidori G, Russo LJ, Vilagut G, Almansa J, Arbabzadeh-Bouchez S, Autonell J, Bernal M, Buist-Bouwman MA, Codony M, Domingo-Salvany A, Ferrer M, Joo SS, Martinez-Alonso M, Matschinger H, Mazzi F, Morgan Z, Morosini P, Palacin C, Romera B, Taub N, Vollebergh WA: Disability and quality of life impact of mental disorders in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand 2004; 420:38–46Google Scholar
5. Rapaport MH, Clary C, Fayyad R, Endicott J: Quality-of-life impairment in depressive and anxiety disorders. Am J Psychiatry 2005; 162:1171–1178Google Scholar
6. Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A: Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry 2003; 60:837–844Google Scholar
7. Stein MB, Fuetsch M, Muller N, Höfler M, Lieb R, Wittchen HU: Social anxiety disorder and the risk of depression: a prospective community study of adolescents and young adults. Arch Gen Psychiatry 2001; 58:251–256Google Scholar
8. Bittner A, Goodwin RD, Wittchen HU, Beesdo K, Hofler M, Lieb R: What characteristics of primary anxiety disorders predict subsequent major depressive disorder? J Clin Psychiatry 2004; 65:618–626Google Scholar
9. LeDoux JE: Emotional circuits in the brain. Annu Rev Neurosci 2000; 23:155–184Google Scholar
10. Davis M. Functional neuroanatomy of anxiety and fear: a focus on the amygdala, in Neurobiology of Mental Illness. Edited by Charney DS, Nestler EJ, Bunney BS. New York, Oxford University Press, 1999, pp 463–474Google Scholar
11. Armony JL, LeDoux JE: How the brain processes emotional information. Ann N Y Acad Sci 1997; 821:259–270Google Scholar
12. Wilensky AE, Schafe GE, LeDoux JE: The amygdala modulates memory consolidation of fear-motivated inhibitory avoidance learning but not classical fear conditioning. J Neurosci 2000; 20:7059–7066Google Scholar
13. Rauch SL, Shin LM, Wright CI: Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci 2003; 985:389–410Google Scholar
14. Cannistraro PA, Rauch SL: Neural circuitry of anxiety: evidence from structural and functional neuroimaging studies. Psychopharmacol Bull 2003; 37:8–25Google Scholar
15. Adolphs R, Tranel D, Damasio AR: The human amygdala in social judgment. Nature 1998; 393:470–474Google Scholar
16. Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR: A mechanism for impaired fear recognition after amygdala damage. Nature 2005; 433:68–72Google Scholar
17. Whalen PJ, Kagan J, Cook RG, Davis FC, Kim H, Polis S, McLaren DG, Somerville LH, McLean AA, Maxwell JS, Johnstone T: Human amygdala responsivity to masked fearful eye whites. Science 2004; 306:2061Google Scholar
18. Davidson RJ, Irwin W: The functional neuroanatomy of emotion and affective style. Trends Cogn Sci 1999; 3:11–21Google Scholar
19. Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ: A differential neural response in the human amygdala to fearful and happy facial expressions. Nature 1996; 383:812–815Google Scholar
20. Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA: Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 1998; 18:411–418Google Scholar
21. Birbaumer N, Grodd W, Diedrich O, Klose U, Erb M, Lotze M, Schneider F, Weiss U, Flor H: fMRI reveals amygdala activation to human faces in social phobics. NeuroReport 1998; 9:1223–1226Google Scholar
22. Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG: Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry 2002; 59:1027–1034Google Scholar
23. Furmark T, Tillfors M, Garpenstrand H, Marteinsdottir I, Langstrom B, Oreland L, Fredrikson M: Serotonin transporter polymorphism related to amygdala excitability and symptom severity in patients with social phobia. Neurosci Lett 2004; 362:189–192Google Scholar
24. Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME: Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry 2006; 59:424–429Google Scholar
25. Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK: Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry 2000; 47:769–776Google Scholar
26. Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL: A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry 2005; 62:273–281Google Scholar
27. Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson DA, Whalen PJ, Casey BJ: Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry 2001; 58:1057–1063Google Scholar
28. Wright CI, Martis B, McMullin K, Shin LM, Rauch SL: Amygdala and insular responses to emotionally valenced human faces in small animal specific phobia. Biol Psychiatry 2003; 54:1067–1076Google Scholar
29. Cannistraro PA, Wright CI, Wedig MM, Martis B, Shin LM, Wilhelm S, Rauch SL: Amygdala responses to human faces in obsessive-compulsive disorder. Biol Psychiatry 2004; 56:916–920Google Scholar
30. Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL: Inhibited and uninhibited infants “Grown Up”: adult amygdalar response to novelty. Science 2003; 300:1952–1953Google Scholar
31. Endler NS, Kocovski NL: State and trait anxiety revisited. J Anxiety Disord 2001; 15:231–245Google Scholar
32. Hirschfeld RM, Klerman GL, Lavori P, Keller MB, Griffith P, Coryell W: Premorbid personality assessments of first onset of major depression. Arch Gen Psychiatry 1989; 46:345–350Google Scholar
33. Andrews G, Slade T, Issakidis C: Deconstructing current comorbidity: data from the Australian national survey of mental health and well-being. Br J Psychiatry 2002; 181:306–314Google Scholar
34. Kendler KS, Myers J, Prescott CA: The etiology of phobias: an evaluation of the stress-diathesis model. Arch Gen Psychiatry 2002; 59:242–248Google Scholar
35. Reiss S, Peterson RA, Gursky DM, McNally RJ: Anxiety sensitivity, anxiety frequency, and the prediction of fearfulness. Behav Res Ther 1986; 24:1–8Google Scholar
36. Schmidt NB, Lerew DR, Jackson RJ: Prospective evaluation of anxiety sensitivity in the pathogenesis of panic: replication and extension. J Abnorm Psychol 1999; 108:532–537Google Scholar
37. Hayward C, Killen JD, Kraemer HC, Taylor CB: Predictors of panic attacks in adolescents. J Am Acad Child Adolesc Psychiatry 2000; 39:207–214Google Scholar
38. Wilson KA, Hayward C: A prospective evaluation of agoraphobia and depression symptoms following panic attacks in a community sample of adolescents. J Anxiety Disord 2005; 19:87–103Google Scholar
39. Phillips ML, Drevets WC, Rauch SL, Lane R: Neurobiology of emotion perception, I: the neural basis of normal emotion perception. Biol Psychiatry 2003; 54:504–514Google Scholar
40. Adolphs R: Cognitive neuroscience of human social behaviour. Nat Rev Neurosci 2003; 4:165–178Google Scholar
41. Augustine JR: Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev 1996; 22:229–244Google Scholar
42. Phillips ML, Williams LM, Heining M, Herba CM, Russell T, Andrew C, Bullmore ET, Brammer MJ, Williams SC, Morgan M, Young AW, Gray JA: Differential neural responses to overt and covert presentations of facial expressions of fear and disgust. Neuroimage 2004; 21:1484–1496Google Scholar
43. Phan KL, Wager T, Taylor SF, Liberzon I: Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 2002; 16:331–348Google Scholar
44. Buchel C, Morris J, Dolan RJ, Friston KJ: Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron 1998; 20:947–957Google Scholar
45. Gorno-Tempini ML, Pradelli S, Serafini M, Pagnoni G, Baraldi P, Porro C, Nicoletti R, Umita C, Nichelli P: Explicit and incidental facial expression processing: an fMRI study. Neuro Image 2001; 14:465–473Google Scholar
46. Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR: Serotonin transporter genetic variation and the response of the human amygdala. Science 2002; 297:401–403Google Scholar
47. Paulus MP, Feinstein JS, Castillo G, Simmons AN, Stein MB: Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Arch Gen Psychiatry 2005; 62:282–288Google Scholar
48. Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR: A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry 2005; 62:146–152Google Scholar
49. Puce A, Allison T, Gore JC, McCarthy G: Face-sensitive regions in human extrastriate cortex studied by functional MRI. J Neurophysiol 1995; 74:1192–1199Google Scholar
50. Hoffman EA, Haxby JV: Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nat Neurosci 2000; 3:80–84Google Scholar
51. Loffler G, Yourganov G, Wilkinson F, Wilson HR: fMRI evidence for the neural representation of faces. Nat Neurosci 2005; 8:1386–1390Google Scholar
52. Williams LM, Kemp AH, Felmingham K, Barton M, Olivieri G, Peduto A, Gordon E, Bryant RA: Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. Neuroimage 2006; 29:347–357Google Scholar
53. Spielberger CD: Manual for State-Trait Anxiety Inventory. Palo Alto, Calif, Consulting Psychologists Press, 1983Google Scholar
54. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM–IV Axis I Disorders, Clinician Version (SCID-I). Arlington, Va, American Psychiatric Publishing, 1997Google Scholar
55. Costa PT, Jr, McCrae RR: NEO PI-R Professional Manual. Odessa, Fla, Psychological Assessment Resources, Inc., 1992Google Scholar
56. Reznick JS, Hegeman IM, Kaufman ER, Woods SW, Jacobs M: Retrospective and concurrent self-report of behavioral inhibition and their relation to adult mental health. Dev Psychopathol 1992; 4:301–321Google Scholar
57. Bohn MJ, Babor TF, Kranzler HR: The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol 1995; 56:423–432Google Scholar
58. Skinner HA: The Drug Abuse Screening Test. Addict Behav 1982; 7:363–371Google Scholar
59. Cox RW: Software for analysis and visualization of functional magnetic neuroimages. Comput Biomed Res 1996; 29:162–173Google Scholar
60. Friston KJ, Frith CD, Turner R, Frackowiak RS: Characterizing evoked hemodynamics with fMRI. NeuroImage 1995; 2:157–165Google Scholar
61. Cohen MS: Parametric analysis of fMRI data using linear systems methods. NeuroImage 1997; 6:93–103Google Scholar
62. Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey LH, Kochunov PV, Nickerson D, Mikiten SA, Fox PT: Automated Talairach atlas for functional brain mapping. Hum Brain Mapp 2000; 10:120–131Google Scholar
63. Cools R, Calder AJ, Lawrence AD, Clark L, Bullmore E, Robbins TW: Individual differences in threat sensitivity predict serotonergic modulation of amygdala response to fearful faces. Psychopharmacol (Berl) 2005; 180:670–679Google Scholar
64. Killgore WD, Yurgelun-Todd DA: Social anxiety predicts amygdala activation in adolescents viewing fearful faces. Neuroreport 2005; 16:1671–1675Google Scholar
65. Bertolino A, Arciero G, Rubino V, Latorre V, De Candia M, Mazzola V, Blasi G, Caforio G, Hariri A, Kolachana B, Nardini M, Weinberger DR, Scarabino T: Variation of human amygdala response during threatening stimuli as a function of 5-HTTLPR genotype and personality style. Biol Psychiatry 2005; 57:1517–1525Google Scholar
66. Straube T, Kolassa IT, Glauer M, Mentzel HJ, Miltner WH: Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study. Biol Psychiatry 2004; 56:921–930Google Scholar
67. Botvinick M, Jha AP, Bylsma LM, Fabian SA, Solomon PE, Prkachin KM: Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. Neuroimage 2005; 25:312–319Google Scholar
68. Simmons A, Matthews SC, Stein MB, Paulus MP: Anticipation of emotionally aversive visual stimuli activates right insula. Neuroreport 2004; 15:2261–2265Google Scholar
69. Critchley H: Emotion and its disorders. Br Med Bull 2003; 65:35–47Google Scholar
70. Critchley HD, Rotshtein P, Nagai Y, O’Doherty J, Mathias CJ, Dolan RJ: Activity in the human brain predicting differential heart rate responses to emotional facial expressions. Neuroimage 2005; 24:751–762Google Scholar
71. Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ: Neural systems supporting interoceptive awareness. Nat Neurosci 2004; 7:189–195Google Scholar
72. Paulus MP, Stein MB: An insular view of anxiety. Biol Psychiatry 2006; 60:383–387Google Scholar
73. Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Klein S, Grusser SM, Flor H, Schumann G, Mann K, Buchel C: Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci 2005; 8:20–21Google Scholar
74. Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR: 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci 2005; 8:828–834Google Scholar
75. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 2005; 102:9673–9678Google Scholar