Increased Brain Activation During Working Memory in Cognitively Intact Adults With the APOE ε4 Allele
Abstract
Objective: Altered patterns of brain activity during cognitive tasks have been demonstrated using functional magnetic resonance imaging (fMRI) in mild cognitive impairment and Alzheimer’s disease. However, there have been few studies of adults at genetic risk for Alzheimer’s disease prior to the onset of symptoms. The purpose of this study was to determine whether brain activation patterns associated with working memory differ as a function of apolipoprotein E (APOE) genotype in cognitively intact adults. Method: Participants were cognitively intact, healthy adults who completed genotyping, comprehensive neuropsychological testing, and structural and functional neuroimaging. Twenty-two participants had the APOE ε3/ε3 genotype, and 13 participants had the APOE ε3/ε4 genotype. The study employed an auditory verbal N-back task to probe working memory-related brain activity. Results: The ε3/ε3 and ε3/ε4 groups did not differ in demographic characteristics, cognitive ability, mood, or in-scanner task performance. The ε3/ε4 group showed greater activity during working memory in the medial frontal and parietal regions bilaterally and in the right dorsolateral prefrontal cortex. There were no regions in which the ε3/ε3 group showed greater activation than the ε3/ε4 group. Conclusions: These results indicate that differences in brain activity are evident in cognitively intact individuals who are at risk for late-onset Alzheimer’s disease by virtue of their APOE allele status. As neuroprotective interventions become available, early detection will increase in importance. The combination of genetic and functional neuroimaging strategies may prove useful for monitoring individuals at risk for Alzheimer’s disease before the onset of cognitive symptoms.
Early detection of Alzheimer’s disease is taking on increasing importance as treatments are developed to prevent or delay the onset of symptoms. It is therefore necessary to identify and treat individuals in the prodromal stages of the disease, such as mild cognitive impairment. Clinical trial data indicate positive effects of cholinesterase inhibition in patients with mild cognitive impairment (1) . Given that the disease process likely begins years prior to the onset of observable cognitive problems (2) , detection at the earliest possible time point may yield new options for treatment and prevention. Unfortunately, there is currently no valid method to identify asymptomatic adults who will go on to develop late-onset Alzheimer’s disease. The combination of genetic, neuropsychological, and neuroimaging strategies may prove useful in this regard.
Apolipoprotein E (APOE) is the susceptibility gene most clearly linked to late-onset Alzheimer’s disease. One APOE ε allele doubles the risk of Alzheimer’s disease, and two alleles confer a fivefold increase in risk (3) . Other genes likely interact with APOE, and factors in addition to genotype are required to predict risk for late-onset Alzheimer’s disease (2) . For example, research suggests that memory deficits and medial temporal atrophy occur in otherwise asymptomatic ε-positive adults (4 , 5) and may help predict risk for Alzheimer’s disease. In addition, positron emission tomography (PET) studies of asymptomatic ε-positive adults show hypometabolism of the bilateral parietal, temporal and prefrontal regions, and the cingulate—areas that are linked to Alzheimer’s disease (6 , 7) . To date, few functional magnetic resonance imaging (fMRI) studies of cognitively intact adults at genetic risk for Alzheimer’s disease have been conducted (8 – 13) , and the existing studies are equivocal with regard to a number of issues. Results of existing studies differ with regard to whether brain activity is increased or decreased in at-risk individuals and whether the findings are specific to certain cognitive abilities or more global. It is also unclear how the alterations in brain activity in genetically at-risk individuals correspond to those observed in patients with mild cognitive impairment or Alzheimer’s disease. Nonetheless, these studies suggest that regionally specific differences in brain activation are present in cognitively intact ε-positive adults relative to ε-negative adults.
We previously reported reduced activation of frontoparietal circuitry during a two-back working memory task in patients with mild cognitive impairment relative to healthy comparison subjects (14) . Working memory, which involves the short-term online storage and manipulation of information, is affected in Alzheimer’s disease and other dementias (15) . Deficits in working memory have also been demonstrated using specialized testing in otherwise cognitively intact APOE ε carriers (16) . To our knowledge, except for preliminary work (9) , there are no fMRI studies examining working memory circuitry as a function of APOE genotype in cognitively intact individuals. Therefore, we examined activity during a two-back working memory task to determine whether different brain activation patterns are observable in ε-positive adults versus ε-negative adults in the absence of cognitive impairment.
Method
Participants
We recruited 40 healthy, right-handed, cognitively intact adults from our local community. Inclusion criteria were as follows: aged 25 to 75 years, 12 or more years of education or General Education Development degree, fluency in English, right-handedness, and normal hearing. Exclusion criteria were presence of a neurological, psychiatric, or medical condition known to affect brain structure or function; history of traumatic brain injury with loss of consciousness exceeding 5 minutes; psychoactive medications, including cholinergic, anticholinergic, and antidepressant medications; current or past dependence on alcohol or drugs; and contraindication for magnetic resonance (MR) scanning (e.g., ferromagnetic metal in the body, pacemaker). After complete description of the study to the subjects, written informed consent was obtained.
Neuropsychological Assessment
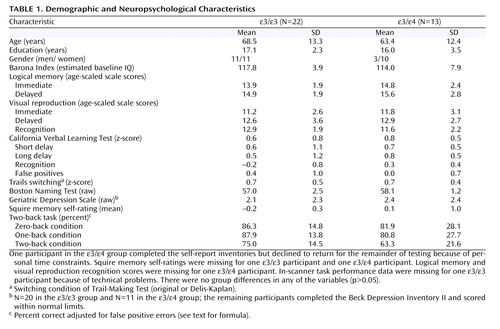
Neuropsychological testing included measures of learning and memory (California Verbal Learning Test [17] or California Verbal Learning Test-II [18] ) and logical memory and visual reproduction subtests of the Wechsler Memory Scale-III [19] ), cognitive flexibility (switching condition of the Trail-Making Test [ 20 , 21 ]), and confrontation naming (Boston Naming Test [22] ). Also included were self-report measures of depression (Geriatric Depression Scale [23] ) and memory functioning (Squire Memory Self-Rating Scale [24] ). The Barona Index (25) , a demographics-based algorithm, was used to estimate baseline intellectual ability. Testing was carried out by highly trained technicians and postdoctoral fellows under the supervision of a faculty neuropsychologist. For tests with alternate forms (California Verbal Learning Test, Trail-Making Test), there were no group differences in the forms used (p>0.05). Neuropsychological and MR data were acquired by examiners blind to APOE status.
MRI
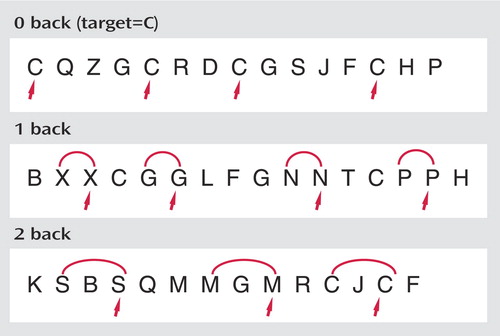
All scanning was completed on a GE LX 1.5-Tesla Horizon scanner with echo speed gradients (TR=2500 msec, TE=40 msec, field of view=24 cm, number of excitations=1) yielding 29 contiguous 5-mm sagittal slices in a 64 x 64 matrix with an in-plane resolution of 3.75 mm 2 . The working memory task used for fMRI stimulation is an auditory N-back paradigm adapted from Jonides and colleagues (26) that we have used in our prior studies (14 , 27 , 28) . In this task, shown in Figure 1 , a different consonant (except L, W, and Y) is presented once every 3 seconds, with certain letters repeated at preset intervals. In separate zero-, one-, and two-back conditions, the individual presses a button with the thumb of their dominant (right) hand when the letter being presented matches a target letter or matches the letter that is one or two positions back in the list. They press an adjacent button with their right index finger for nonmatches. The one-back condition primarily requires vigilance, and the two-back condition makes additional demands involving working memory. The zero-back condition functions as a low-level control. Three 27-second epochs for each of the three task conditions are presented in pseudorandom order. There are a total of 27 items in each of the zero-, one-, and two-back conditions, including seven targets and 20 foils. An accuracy score, adjusted for false positive responding, is calculated for each condition using the following formula: (Correct –[0.35 x FP]/ 7) x 100. Using this formula, the adjusted accuracy score would be 0 if a person responded positively to every item. MR-compatible headphones (Resonance Technology, Inc., Northridge, Calif.) and foam ear inserts were used to minimize extraneous noise in the scanner. Participants were provided with detailed instructions regarding the importance of minimizing head motion in the scanner, and a foam pillow was used to stabilize head position. T 1 - and T 2 -weighted structural scans were also obtained, and a board-certified neuroradiologist (A.C.M.) reviewed the scans to ascertain whether any participant had unexpected brain abnormalities.
fMRI Preprocessing Procedures
Blood-oxygen-level-dependent echoplanar scan data were reconstructed, reviewed for data quality, and spatially realigned using standard preprocessing procedures as implemented in statistical parametric mapping (SPM99; Wellcome Department of Cognitive Neurology, University College, London). Data were normalized to the Montreal Neurological Institute (MNI) standard atlas using a 12-parameter affine approach followed by nonlinear warping by spatial basis functions. Data were resampled to 2 mm 3 isotropic voxels and spatially smoothed to 10 mm, full width at half maximum.
Analyses
Statistical parametric mapping was performed on a voxel-by-voxel basis using general linear model and random effects procedures as implemented in SPM-2. The contrast for the two-back condition relative to the zero-back condition was selected for this study in order to derive maximum statistical power by comparing the highest level working memory condition with the low level control task. To test for hypothesized differences in brain activity as a function of genotype, the contrast was entered into an analysis of variance in SPM-2, with the group (ε3/ε4 versus ε3/ε3) as the independent variable and task-related signal change at each voxel as the dependent variable. Given our focus on working memory circuitry, including bilateral frontal and parietal regions, threshold correction for a whole brain search would have been overly conservative. However, we employed a hierarchical strategy that is somewhat more stringent than those employed in most prior studies of this kind, in which regions were required to be significant at 0.05 at both the cluster level and 0.001 at the voxel level. While both levels incorporate a signal intensity threshold, the inclusion of a cluster level correction places an additional spatial constraint on significance testing.
Results
Genotyping
Genotyping was performed in the Molecular Genetics Diagnostic Laboratory at Dartmouth-Hitchcock Medical Center using standard techniques (29 , 30) . Twenty-two participants had the APOE ε3/ε3 genotype, and 13 participants had the ε3/ε4 genotype. Data from four additional ε2/ε3 individuals and one ε2/ε4 individual were excluded from this study because of the small number of people with these particular genotypes and the potential protective effect of the ε2 allele (3) .
Demographic and Cognitive Characteristics
There were no significant differences between the ε3/ε3 and ε/ε groups in demographic characteristics ( Table 1 ). Because group gender distributions approached significance, analyses of genotype effects were also run with gender as a covariate. Group means for all neuropsychological tests, including measures of verbal and visual learning and memory, executive ability, language, and estimated baseline intellect, were in the average range or above, and there were no group differences (all p>0.05). Memory self-ratings also showed no group differences (p>0.05). All participants scored within normal limits on the mood self-ratings, and there were no group differences in emotional status (p>0.05). In addition, there were no group differences in adjusted accuracy scores on any of the three conditions of the in-scanner working memory task (all p>0.05).
Brain Imaging Results
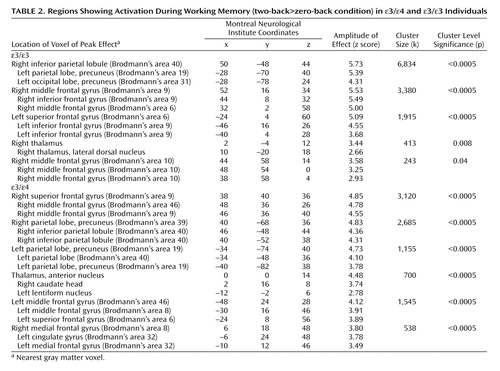
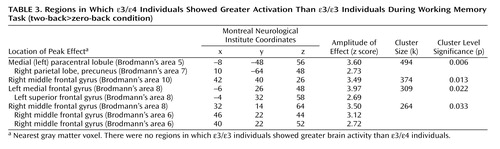
Separate analyses for each group showed activation of distributed cortical and subcortical regions, predominantly in the bilateral frontoparietal and thalamic areas ( Figure 2 , Table 2 ). The between-group analysis of variance demonstrated greater activity in the ε/ε group than in the ε/ε group in the medial frontal and parietal regions bilaterally and in the right dorsolateral prefrontal cortex (Brodmann’s areas 6, 8, and 10; Table 3 , Figure 3 ). There were no regions in which the ε/ε group showed greater activation than the ε/ε group. The pattern of results, including the specific brain regions showing group differences, remained the same after covarying for gender.
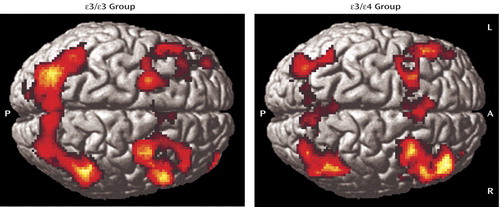
a Both groups showed activity in distributed frontal and parietal regions as expected for a working memory task. Images are shown in neurological convention at a threshold of p<0.01 and three contiguous voxels for display purposes. (See Table 2 and text for analyses and a detailed description of the results.)
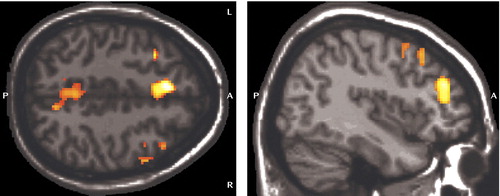
a There were no regions in which the ε/ε group showed greater activation than the ε3/ε4 group. Image is shown in neurological convention at a threshold of p< 0.01 and three contiguous voxels for display purposes. (See text for results of statistical comparisons and Table 3 for detailed results.)
We employed a neuropsychological index approach to identify any individuals with early cognitive impairment who might have had an undue effect on the findings. We selected eight cognitive measures on an a priori basis for this index, including California Verbal Learning Test (total learning), short delay and long delay, logical memory immediate and delayed recall, visual reproduction immediate and delayed recall, Trail-Making Test (switching condition), and Boston Naming Test (total accuracy). Participants were considered to be impaired on the index if they scored 1.5 standard deviations below published age-appropriate normative data on two or more measures or two standard deviations below the norm on one or more measures. No participant in either group scored in the abnormal range on this index. However, two participants in the ε/ε group lacked data on some of the key measures, and thus the analyses were repeated after excluding these two individuals. The ε/ε participants showed greater activation in the same brain regions as in the original analysis, except that the statistical significance of the finding for the right middle frontal gyrus dropped from 0.03 to 0.18 in the reduced sample, and an additional area of increased activation in the ε/ε group emerged in the left anterior cingulate (Brodmann’s area 32, MNI coordinates: –8, 44, 16; amplitude of effect: z=2.94; cluster size: k=388; cluster level significance: p=0.02). In addition, a single region emerged that showed greater activity in the ε/ε participants than in the ε/ε participants (left cuneus, Brodmann’s area 18, MNI coordinates: –12, –94, 16; amplitude of effect: z=3.38; cluster size: k=386; cluster level significance: p=0.01). Despite these isolated changes, the overall pattern of findings, with relatively greater activity in the ε/ε group than in the ε/ε group, was maintained after excluding the two subjects in the ε/ε group who lacked data on a number of key measures.
Discussion
In this study, cognitively intact adults with the APOE ε/ε genotype showed greater brain activation during working memory than demographically matched ε/ε individuals. Areas of greater activation in the ε/ε group included medial prefrontal and parietal regions bilaterally as well as the right dorsolateral prefrontal cortex. There were no areas in which ε/ε individuals consistently showed greater activation than ε/ε individuals. These differences in brain activity were observed despite the fact that participants in both groups were cognitively intact and did not differ in performance on the in-scanner task.
Taken together with prior fMRI research investigating the role of APOE in various cognitive domains (9 – 13) , these results suggest that the presence of an ε allele is associated with upregulation of activity in episodic, semantic, and working memory in cognitively intact adults. In the case of semantic memory, regions of decreased activation have also been observed (11 , 12) . The increased activation might reflect compensatory recruitment of neural resources that helps maintain normal cognition in individuals with genetic risk for Alzheimer’s disease (10) . If this is the case, then cognitive symptoms may emerge when this additional activation can no longer be supported after a critical threshold of brain pathology is reached. Bookheimer and colleagues (10) previously reported increased brain activity during episodic memory processing in APOE ε carriers. In the study by Bookheimer and colleagues, 2-year follow-up of a subsample of participants indicated that baseline brain activation patterns predicted memory decline over time. Overall, these findings are consistent with longitudinal neuropsychological research that has demonstrated decline in learning and memory in older ε-positive adults prior to the onset of Alzheimer’s disease or mild cognitive impairment (4 , 5) . It will be important to assess the compensatory hypothesis through continued longitudinal research combining structural and functional neuroimaging approaches with genetics and neuropsychological testing.
Another possible explanation for the observed increase in activation in the ε-positive group is that it represents an early abnormality of brain function, such as de-differentiation or decreased inhibition in otherwise intact individuals. Prior research has demonstrated hypometabolism in distributed cortical regions in cognitively intact ε-positive individuals relative to ε-negative individuals (7) , including some but not all of the areas that showed upregulated activity in our study. This raises the question regarding whether hypometabolism may also contribute to the relative increase in activation observed in the ε-positive group during task performance, although further research integrating PET and fMRI would be necessary to determine the relationship of metabolic rate to blood-oxygen-level-dependent activation in these individuals. It is also possible that early structural and metabolic brain abnormalities and compensatory processes coexist or interact to produce the observed activation patterns.
Further research is needed to determine whether altered brain activation patterns are specific to working, episodic, and semantic memory domains or can be observed more globally. One study showed no differences in pattern of activation between ε-positive and ε-negative individuals on a digit repetition test, although both groups showed increased activation of the frontal, parietal, and temporal regions as a function of condition difficulty (8) . The authors suggested that increases in brain activation in ε-positive adults may be specific to memory processing rather than globally present for demanding cognitive activities. However, APOE genotype has been implicated in attentional processes in other research (31) and has been found to relate to the risk or severity of cognitive problems and disability in a number of conditions, such as cardiovascular disease (32) , traumatic brain injury (33) , multiple sclerosis (34) , and cancer chemotherapy (35) . Overall, APOE may play a broader role in response to brain injury (36) , so the possibility of a more general relation between APOE status and cognition-related brain activity in cognitively intact adults warrants further investigation.
A limitation of our study is that the ε/ε group had a preponderance of men, although the group gender distributions were nonsignificant and covarying for gender did not affect the findings. Nonetheless, future research should determine whether gender modifies the relationship between APOE status and brain activity patterns in cognitively intact adults. In addition, other variables such as promoter polymorphisms, genes other than APOE, and environmental factors are likely to be important (37 , 38) , and a polygenic approach may be required. Both participant groups in this study were highly educated and showed average to above average cognitive ability. Research in less educated samples will be necessary to evaluate the potential role of cognitive reserve in the present findings and to enhance generalizability to the general population.
Our recruitment strategy also merits discussion. We could have adopted an individual-matching strategy to form the groups in this study, but that would have necessitated the exclusion of some with ε homozygotes, who are more numerous than ε/ε individuals in the general population. We opted instead to keep the sample intact as a consecutive series of participants meeting inclusion criteria. It is possible that group differences would have been less apparent with an individual-matching strategy. Although most prior studies employed a series of participants, which was done in our study, one recent study that used an individual matching approach also demonstrated increased brain activity in ε-positive individuals (13) . A possible advantage of our study design is that we chose to compare genetically homogeneous ε/ε and ε/ε groups, and thus findings could not be attributed to the inclusion of one or two individuals with ε/ε, ε/ε, or other rarer genotypes that could influence findings.
In our study, the ε/ε group showed slightly, although not significantly, lower accuracy on the in-scanner activation task. It is possible that this difference would have reached significance with a larger sample, and future research should stratify by task performance. We chose an a priori basis to examine the two-back relative to the zero-back condition to achieve maximal statistical power in this initial study. In ongoing research, we are collecting data on a more difficult three-back task in a larger sample in order to examine individual stepwise contrasts (1>0, 2>1, 3>2) to assess component cognitive processes and parametric task effects.
Our results indicate that differences in brain activity are evident in cognitively intact individuals who are at risk for late-onset Alzheimer’s disease by virtue of their APOE genotype. In the future, functional neuroimaging could potentially be used to help improve prognostic accuracy and monitor effects of treatments as they become available. Available data suggest that patients with mild cognitive impairment and Alzheimer’s disease show decreased activation of medial temporal regions during episodic memory (39 – 42) and decreased activation of frontoparietal circuitry during working memory (14) . Although these data might lead to speculation that ε-positive individuals may also show decreased activation relative to ε-negative individuals, our findings and those of related studies (9 , 10) suggest the opposite. Further research is needed to determine at what point brain activity may be increased, the mechanisms that govern subsequent decline, and how this relates to the emergence of cognitive symptoms.
1. Salloway S, Ferris S, Kluger A, Goldman R, Griesing T, Kumar D, Richardson S: Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology 2004; 63:651–657Google Scholar
2. Small GW: Use of neuroimaging to detect early brain changes in people at genetic risk for Alzheimer’s disease. Adv Drug Deliv Rev 2002; 54:1561–1566Google Scholar
3. Mayeux R: Epidemiology of neurodegeneration. Ann Rev Neurosci 2003; 26:81–104Google Scholar
4. Baxter LC, Caselli RJ, Johnson SC, Reiman EM, Osborne D: Apolipoprotein E ε affects new learning in cognitively normal individuals at risk for Alzheimer’s disease. Neurobiol Aging 2003; 24:947–952Google Scholar
5. Caselli RJ, Reiman EM, Osborne D, Hentz JG, Baxter LC, Hernandez JL, Alexander GG: Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE ε allele. Neurology 2004; 62:1990–1995Google Scholar
6. Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D: Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med 1996; 334:752–758Google Scholar
7. Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J: Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci USA 2004; 101:284–289Google Scholar
8. Burggren AC, Small GW, Sabb FW, Bookheimer SY: Specificity of brain activation patterns in people at genetic risk for Alzheimer disease. Am J Geriatr Psychiatry 2002; 10:44–51Google Scholar
9. Petrella J, Lustig C, Bucher L, Jha A, Doraiswamy P: Prefrontal activation patterns in subjects at risk for Alzheimer disease. Am J Geriatr Psychiatry 2002; 10:112–113Google Scholar
10. Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW: Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med 2000; 343:450–456Google Scholar
11. Smith CD, Andersen AH, Kryscio RJ, Schmitt FA, Kindy MS, Blonder LX, Avison MJ: Altered brain activation in cognitively intact individuals at high risk for Alzheimer’s disease. Neurology 1999; 53:1391–1396Google Scholar
12. Smith CD, Andersen AH, Kryscio RJ, Schmitt FA, Kindy MS, Blonder LX, Avison MJ: Women at risk for AD show increased parietal activation during a fluency task. Neurology 2002; 58:1197–1202Google Scholar
13. Bondi MW, Houston WS, Eyler LT, Brown GG: fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer’s disease. Neurology 2005; 64:501–508Google Scholar
14. Saykin AJ, Wishart HA, Rabin LA, Flashman LA, McHugh TL, Mamourian AC, Santulli RB: Cholinergic enhancement of frontal lobe activity in mild cognitive impairment. Brain 2004; 127:1574–1583Google Scholar
15. Kensinger EA, Shearer DK, Locascio JJ, Growdon JH, Corkin S: Working memory in mild Alzheimer’s disease and early Parkinson’s disease. Neuropsychology 2003; 17:230–239Google Scholar
16. Rosen VM, Bergeson JL, Putnam K, Harwell A, Sunderland T: Working memory and apolipoprotein E: what’s the connection? Neuropsychologia 2002; 40:2226–2233Google Scholar
17. Delis DC, Kramer JH, Kaplan E, Ober BA: California Verbal Learning Test: Adult Version Research Edition Manual. San Antonio, Psychological Corporation, 1987Google Scholar
18. Delis DC, Kramer JH, Kaplan E, Ober BA: California Verbal Learning Test-2nd ed: Adult Version Manual. San Antonio, Psychological Corporation, 2000Google Scholar
19. Wechsler D: Wechsler Memory Scale, 3rd ed: WMS-III Administration and Scoring Manual. San Antonio, Tex., Psychological Corporation, 1997Google Scholar
20. Spreen O, Strauss E: A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York, Oxford University Press, 1998Google Scholar
21. Delis D, Kaplan E: Delis-Kaplan Executive Function System. San Antonio, Texas, Psychological Corporation, 2001Google Scholar
22. Goodglass H, Kaplan E, Barresi B: Boston Diagnostic Aphasia Examination, 3rd ed. Philadelphia, Lippincott Williams & Wilkins, 2001Google Scholar
23. Yesavage JA, Brink TL, Lose TL, Lum O, Huang V, Adey M, Leirer VO: Development and validation of geriatric depression rating scale: a preliminary report. J Psychiatr Res 1982; 17:37–49Google Scholar
24. Squire LR: ECT and memory loss. Am J Psychiatry 1977; 134:997–1001Google Scholar
25. Barona A, Reynolds C, Chastain R: A demographically based index of pre-morbid intelligence for the WAIS-R. J Consult Clin Psychol 1984; 52:885–887Google Scholar
26. Jonides J, Schumacher EH, Smith EE, Lauber E, Awh E, Minoshima S, Koeppe RA: The task load of verbal working memory affects regional brain activation as measured by PET. J Cogn Neurosci 1997; 9:462–475Google Scholar
27. McAllister TW, Saykin AJ, Flashman LA, Sparling MB, Johnson SC, Guerin SJ, Mamourian A, Weaver J, Yanofsky N: Brain activation during working memory 1 month after mild traumatic brain injury: a functional MRI study. Neurology 1999; 53:1300–1308Google Scholar
28. Wishart HA, Saykin AJ, McDonald BC, Mamourian AC, Flashman LA, Schuschu KR, Ryan KA, Fadul CE, Kasper LH: Brain activation patterns associated with working memory in relapsing-remitting MS. Neurology 2004; 62:234–238Google Scholar
29. Reymer W, Groenemeyer V, Van De Burg R, Kastelin J: Apolipoprotein E genotyping on agarose gels. Clin Chem 1995; 41:1046–1047Google Scholar
30. Koch W, Ehrenhaft A, Greisser K, Pfeufer A, Muller J, Schomig A, Kastrati A: Taqman systems for genotyping of disease-related polymorphisms present in the gene encoding apolipoprotein E. Clin Chem Lab Med 2002; 40:1123–1131Google Scholar
31. Parasuraman R, Greenwood PM, Sunderland T: The apolipoprotein E gene, attention, and brain function. Neuropsychology 2002; 16:254–274Google Scholar
32. Smith JD: Apolipoproteins and aging: emerging mechanisms. Ageing Res Rev 2002; 1:345–365Google Scholar
33. Nathoo N, Chetty R, van Dellen JR, Barnett GH: Genetic vulnerability following traumatic brain injury: the role of apolipoprotein E. Mol Pathol 2003; 56:132–136Google Scholar
34. Chapman J, Vinokurov S, Achiron A, Karussis DM, Mitosek-Szewczyk K, Birnbaum M, Michaelson DM, Korczyn AD: APOE genotype is a major predictor of long-term progression of disability in MS. Neurology 2001; 56:312–316Google Scholar
35. Ahles TA, Saykin AJ, Noll WW, Furstenberg CT, Guerin S, Cole B, Mott LA: The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology 2003; 12:612–619Google Scholar
36. Laskowitz DT, Horsburgh K, Roses AD: Apolipoprotein E and the CNS response to injury. J Cereb Blood Flow Metab 1998; 18:465–471Google Scholar
37. Lahiri DK, Sambamurti K, Bennett DA: Apolipoprotein gene and its interaction with the environmentally driven risk factors: molecular, genetic and epidemiological studies of Alzheimer’s disease. Neurobiol Aging 2004; 25:651–660Google Scholar
38. Laws SM, Hone E, Gandy S, Martins RN: Expanding the association between the APOE gene and the risk of Alzheimer’s disease: possible roles for APOE promoter polymorphisms and alterations in APOE transcription. J Neurochem 2003; 84:1215–1236Google Scholar
39. Kato T, Knopman D, Liu H: Dissociation of regional activation in mild AD during visual encoding: a functional MRI study. Neurology 2001; 57:812–816Google Scholar
40. Machulda MM, Ward HA, Borowski B, Gunter JL, Cha RH, O’Brien PC, Petersen RC, Boeve BF, Knopman D, Tang-Wai DF, Ivnik RJ, Smith GE, Tangalos EG, Jack CR, Jr: Comparison of memory fMRI response among normal, MCI, and Alzheimer’s patients. Neurology 2003; 61:500–506Google Scholar
41. Rombouts SA, Barkhof F, Hoogenraad FG, Sprenger M, Scheltens P: Within-subject reproducibility of visual activation patterns with functional magnetic resonance imaging using multislice echo planar imaging. Magn Reson Imaging 1998; 16:105–113Google Scholar
42. Sperling RA, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rosen BR, Schacter DL, Albert M: fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2003; 74:44–50Google Scholar