Visuospatial Processing and the Function of Prefrontal-Parietal Networks in Autism Spectrum Disorders: A Functional MRI Study
Abstract
Objective: Individuals with autism spectrum disorders typically have normal visuospatial abilities but impaired executive functioning, particularly in abilities related to working memory and attention. The aim of this study was to elucidate the functioning of frontoparietal networks underlying spatial working memory processes during mental rotation in persons with autism spectrum disorders. Method: Seven adolescent males with normal IQ with an autism spectrum disorder and nine age- and IQ-matched male comparison subjects underwent functional magnetic resonance imaging scans while performing a mental rotation task. Results: The autism spectrum disorders group showed less activation in lateral and medial premotor cortex, dorsolateral prefrontal cortex, anterior cingulate gyrus, and caudate nucleus. Conclusions: The finding of less activation in prefrontal regions but not in parietal regions supports a model of dysfunction of frontostriatal networks in autism spectrum disorders.
Individuals with autism spectrum disorders typically have normal or superior visuospatial abilities (1) but show dysfunction in working memory (2) as an aspect of executive functioning capability. Functional magnetic resonance imaging (fMRI) studies of persons with autism spectrum disorders have shown significant metabolic reduction in the prefrontal cortex, specifically in divisions of the anterior cingulate gyrus (3) , a region critically involved in executive function. Reduced activity has also been observed in the posterior cingulate (4) , which has connections to the lateral prefrontal cortex and posterior parietal cortex, regions that aid visuospatial cognition by monitoring sensory events associated with spatial orientation and memory.
Mental rotation is a form of spatial working memory task that consistently activates superior and inferior parietal regions, underlying spatial attention and visuospatial processing, as well as prefrontal areas, such as the dorsolateral prefrontal cortex and anterior cingulate gyrus, reflecting working memory components (5) . The aim of this study was to examine the functioning of frontoparietal networks in autism spectrum disorders. We hypothesized that individuals with autism spectrum disorders would display impaired activation in the anterior cingulate gyrus and lateral prefrontal cortices, consistent with known deficits in attentional and executive functions in autism spectrum disorders.
Method
Seven male adolescents 11–18 years of age (mean=14.7 years, SD=2.9) who met DSM-IV criteria for autistic disorder or Asperger’s disorder were identified through administration of the revised Autism Diagnostic Interview (6) , a structured parent interview, direct child observations, and reports from teachers and therapists. All participants were right-handed, and all had a Wechsler full-scale IQ above 70 (full-scale IQ, mean=114, SD=16.9; performance IQ, mean=118, SD=16.4). Nine healthy right-handed male comparison subjects with no known medical, neurological, or psychiatric disorders were matched to the autism spectrum disorders group by age (mean=15.0 years, SD=1.8) and performance IQ as indicated by the School and College Ability Tests (Career-Wise, Pty., Ltd., Australia). Comparison subjects were screened with the parent form of the Child Behavior Checklist (7) and the Autism Spectrum Screening Questionnaire (8) .
The mental rotation task used three-dimensional Shepard-Metzler-type block shapes as stimuli (angles of rotation: 45 degrees to 180 degrees), with a target stimulus and four test stimuli presented concurrently for 10 seconds. Participants were asked to press one of four buttons on a fiber-optic button console to indicate which of the test stimuli represented the rotated target stimulus. A baseline condition required subjects to indicate which test stimulus was the closest visual match to a target stimulus with no rotation involved. A total of 18 rotation stimuli and 18 baseline stimuli were presented, in alternating blocks of three rotation trials followed by three baseline trials, covering a scanning duration of 6 minutes 36 seconds.
Gradient-echo echo-planar images were acquired on a 3-T GE Signa MR scanner (TR=3000 ms, TE=40 ms, 128×128 matrix at 1.875×1.875 mm resolution, 22 axial slices, 4.5 mm thick, 0.5 mm gap). Functional images were realigned, spatially normalized to Talairach space, and spatially smoothed (8 mm full width at half maximum) using SPM2 (Wellcome Department of Cognitive Neurology, University College, London). For general linear model analysis, each rotation trial was modeled as a discrete event, including both temporal and dispersion derivatives. Realignment parameters were included in the model to account for residual signal variance related to head motion. Random-effects group analysis used single-sample t tests to examine activation in the two groups separately and independent t tests to examine differences between groups. Suprathreshold clusters with a corrected p value of <0.05, in which peak voxels had an uncorrected p value of <0.001, were considered significant.
Results
Mental rotation response times and accuracy did not differ significantly between the two groups (accuracy: autism spectrum disorders group, 49%, SD=15; comparison group: 51%, SD=16; response time: autism spectrum disorders group, 5.51 s, SD=1.55; comparison group: 6.48 s, SD=0.77; Mann-Whitney test, p>0.05). The comparison group showed significant bilateral activation in the inferior parietal cortex, dorsal premotor cortex, supplementary motor area, and anterior cingulate gyrus. Significant activation was also observed in the left dorsolateral prefrontal cortex and in the right inferior and medial frontal gyri, extending into the left caudate head. The autism spectrum disorders group showed only one significant cluster in the right parietal cortex, including the inferior and superior parietal lobule and the supramarginal gyrus.
Between-group random-effects analysis revealed clusters in the frontal lobe that were significantly more active in the comparison group than the autism spectrum disorders group, including the right inferior and medial frontal gyri, incorporating the right caudate head as well as the right dorsal premotor cortex. The most significant was in the anterior cingulate gyrus ( Figure 1 ).
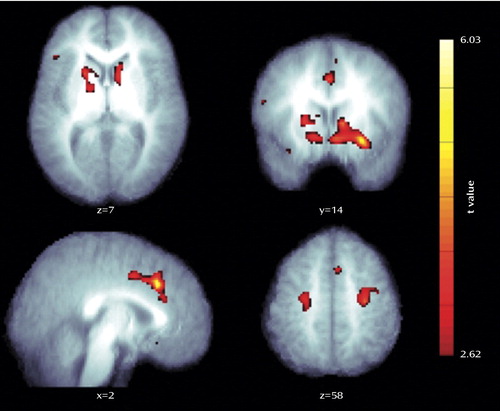
A number of other regions showed a trend, just below our significance threshold, toward greater activation in the comparison group than the autism spectrum disorders group (cluster level uncorrected p<0.05). These included the left dorsolateral prefrontal cortex, right superior parietal lobule, and left premotor cortex. There were no regions in which the autism spectrum disorders group exhibited significantly greater activation than the comparison group.
Discussion
In adolescents with autism spectrum disorders, we observed significantly less activation in cortical and subcortical structures in the frontal lobe, including the anterior cingulate, dorsolateral prefrontal cortex, and caudate nucleus, than in adolescents without such disorders. This finding suggests a disruption of the network underlying key aspects of executive function and working memory. It is also consistent with neuropsychological theories of executive dysfunction in autism spectrum disorders and supports previous neuroimaging findings.
Impaired activation within the head of the caudate nucleus may have the most significance for autism spectrum disorders because of its wide distribution of connections to frontoparietal cortical systems involved in attentional and cognitive control and visuospatial processing. The caudate nucleus is critically connected with the dorsolateral prefrontal cortex and premotor cortex in frontostriatal circuits. Reduced activation in these regions is evidence of disruption to this frontostriatal network in individuals with autism spectrum disorders. These results closely parallel our previous findings (9) of reduced caudate and prefrontal activation in adolescents with attention deficit hyperactivity disorder (ADHD) performing the same mental rotation task.
Regions of the parietal cortex, including the inferior and superior parietal lobules, showed significant activation in both the autism spectrum disorders group and the comparison group. These posterior parietal regions, particularly around the intraparietal sulcus, play a key role in mental rotation (5) and form part of the dorsal visual processing stream that supports perception of spatial properties (10) . Structural differences involving the larger parietal volumes that have been reported in autism spectrum disorders (11) may be expected to result in altered function of the parietal cortex. However, persons with autism spectrum disorders generally have relatively normal or superior spatial abilities (2) . Similarly, our results showed no significant difference between the autism spectrum disorders group and the comparison group on visuospatial task performance, and no difference in activation of the posterior parietal cortex (except for a trend toward reduced activation in part of the right superior parietal lobule). This finding contrasts with what we observed in adolescents with ADHD, who showed significantly worse task performance and reduced activation in the posterior parietal regions during the mental rotation task (10) .
While high-functioning autism and Asperger’s disorder are commonly grouped together, recent research suggests that their neurobiological underpinnings may not be equivalent (12) . Nonetheless, studies such as this one provide an essential starting point for understanding the changes in brain function that accompany autism spectrum disorders. Our findings clearly support a model of dysfunction of frontostriatal networks in these disorders.
1. Caron MJ, Mottron L, Rainville C, Chouinard S: Do high functioning persons with autism present superior spatial abilities? Neuropsychologia 2004; 42:467–481Google Scholar
2. Russell J: Autism as an Executive Disorder. Oxford, UK, Oxford University Press, 1997Google Scholar
3. Ohnishi T, Matsuda H, Hashimoto T, Kunihiro T, Nishikawa M, Uema T, Sasaki M: Abnormal regional cerebral blood flow in childhood autism. Brain 2000; 123:1838–1844Google Scholar
4. Luna B, Minshew NJ, Garver KE, Lazar NA, Thulborn KR, Eddy WF, Sweeney JA: Neocortical system abnormalities in autism: an fMRI study of spatial working memory. Neurology 2002; 59:834–840Google Scholar
5. Cohen MS, Kosslyn SM, Breiter HC, DiGirolamo GJ, Thomas WL, Anderson AK, Bookheimer SY, Rosen BR, Belliveau JW: Changes in cortical activity during mental rotation: a mapping study using functional MRI. Brain 1996; 119:89–100Google Scholar
6. Lord C, Rutter M, Le Couteur A: Autism Diagnostic Interview—Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24:659–685Google Scholar
7. Achenbach TM: Manual for the Child Behavior Checklist/4-18 and 1991 Profile. Burlington, University of Vermont, Department of Psychiatry, 1991Google Scholar
8. Ehlers S, Gillberg C, Wing L: A screening questionnaire for Asperger syndrome and other high-functioning autism spectrum disorders in school age children. J Autism Dev Disord 1999; 29:129–141Google Scholar
9. Silk T, Vance A, Rinehart N, Egan G, O’Boyle M, Bradshaw JL, Cunnington R: Fronto-parietal activation in attention-deficit hyperactivity disorder, combined type: functional magnetic resonance imaging study. Br J Psychiatry 2005; 187:282–283Google Scholar
10. Sakata H, Taira M, Kusunoki M, Murata A, Tanaka Y: The TINS lecture: The parietal association cortex in depth perception and visual control of hand action. Trends Neurosci 1997; 20:350–357Google Scholar
11. Piven J, Arndt S, Bailey J, Andreasen N: Regional brain enlargement in autism: a magnetic resonance imaging study. J Am Acad Child Psychiatry 1996; 35:530–536Google Scholar
12. Rinehart NJ, Bradshaw JL, Brereton AV, Tonge BJ: A clinical and neurobehavioural review of high-functioning autism and Asperger’s disorder. Aust N Z J Psychiatry 2002; 36:762–770Google Scholar



