Interaction Between MAO-A Genotype and Maltreatment in the Risk for Conduct Disorder: Failure to Confirm in Adolescent Patients
Abstract
Objective: Childhood maltreatment is a potent risk factor for subsequent aggressive and criminal behavior. A recent study suggested that the relationship between maltreatment and antisocial behavior may be moderated by a genetic vulnerability conferred by a functional polymorphism in the MAO-A gene. The authors investigated whether these findings would generalize to a clinical cohort of adolescents, examining whether there was a stronger association between maltreatment and conduct disorder severity in patients carrying the low MAO-A activity allele. Method: Male adolescent patients (N=247) entering residential or intensive day treatment for persistent conduct and substance use problems were examined. Conduct disorder severity was indexed by a lifetime count of DSM–IV criteria obtained through structured psychiatric interviews. Maltreatment scores were derived from summing neglect and abuse events reported to have occurred before age 11. Results: Neglect, verbal/psychological abuse, physical abuse, and sexual abuse were prevalent among patients. Although level of maltreatment and lifetime conduct disorder symptoms were significantly correlated, no genetic-environmental interaction with genotype for maltreatment was found. Conclusion: The results of the current study do not support the hypothesis that a polymorphism in the gene encoding MAO-A contributes to the genetic risk for conduct disorder.
Conduct problems such as aggression, lying, and rule violations are common among adolescents. Fortunately, in many cases these behavior patterns are transient and desist by early adulthood. However, some youth show more persistent patterns of aggression and delinquency that often escalate into adult antisocial behavior and comorbid substance use problems (1 – 3) . Early onset of conduct disorder (4 , 5) , early substance use/abuse (6 , 7) , childhood psychiatric problems (e.g., depression, ADHD) (8 , 9) , and parental psychopathology (10 – 12) are features that may identify children with the highest risk for chronic antisocial behavior.
Childhood maltreatment is another potent risk factor for subsequent aggressive and criminal behavior, although many young victims develop normally (13) . Recent interest in the relationship between early child maltreatment and the development of antisocial behavior has been piqued by the intriguing findings of Caspi and colleagues (14) , who suggested that this relationship may be moderated by a genetic vulnerability. Results from the Dunedin longitudinal study of young adult men followed since early childhood (15) have suggested that maltreatment in the “first decade of life” increases the risk for antisocial outcomes (i.e., conduct disorder, aggression, antisocial personality), particularly among individuals carrying a low-activity MAO-A allele, a functional polymorphism in the promoter region of the gene encoding monoamine oxidase A (MAO-A). MAO-A is an enzyme responsible for metabolizing monoamine drugs and neurotransmitters, including dopamine, norepinephrine, and serotonin. Imbalances in the levels of these neurotransmitters have been linked to aggression in both mice (16 – 19) and humans (20 – 22) . Individuals with the rare allele possess relatively lower enzyme activity.
The Dunedin study findings were among the first to suggest a genetic-environmental interaction in the risk for development of psychiatric disorders. The authors concluded that replication of the effect in a clinical sample would be essential to validate the utility of this finding. In this investigation, we attempt to confirm the Caspi et al. findings (14) in a cohort of male adolescents who were entering residential or intensive day treatment for significant conduct and substance use problems. These patients were also participating in large-scale genetic studies aiming to identify chromosomal loci conferring risk for comorbid conduct disorder and substance use disorders (23 , 24) . The patients underwent a comprehensive assessment of abuse and neglect and childhood psychopathology. In addition to examining the relationship between overall levels of childhood maltreatment and severity of adolescent conduct problems, which may or may not vary as a function of MAO-A genotype, we extended the Caspi et al. (14) analyses to include an examination of four individual domains of maltreatment: neglect, verbal/psychological abuse, physical abuse, and sexual abuse.
Method
Subjects
Patients were 247 male adolescents (ages 12–18 years) who were referred to residential or intensive day treatment between 1998 and 2002 for conduct and substance use problems. Most patients (99%) were referred by juvenile justice and social service agencies. Program staff obtained written informed consent from the parent or guardian and assent from the adolescent patient for treatment as well as participation in clinical and genetic research. Admission criteria included significant conduct and substance use problems. Exclusion criteria were current psychosis, mental retardation, current suicidal or homicidal ideation, current intoxication, or current illness that prevented participation in treatment activities. Females were excluded from our cohort in order to make it comparable to the Caspi et al. (14) study. Male patients who were admitted to treatment, but did not remain in treatment for at least 7 days (in order to complete the assessment protocols used in this study) or did not maintain drug-free urine tests before assessment, were not included in the study cohort.
Assessments
Examiners were rigorously trained in the administration of a structured psychiatric assessment before conducting face-to-face interviews with each patient. The National Institute of Mental Health Diagnostic Interview Schedule for Children (DISC–IV) was used to assess conduct disorder symptoms according to DSM-IV criteria. Lifetime symptom counts were used in order to avoid the biases that may arise with past year reports, given that many of the patients had been incarcerated for extended periods prior to admission. Conduct disorder scores were age-corrected on the basis of a sample of 2,067 male adolescents participating in community-based family studies of problem behavior (25) . This method was deemed most appropriate, since the natural increase in symptoms with age that we would expect to see in the general population would be masked in the clinical cohort by the fact that they had been referred for treatment on the basis of their conduct disorder severity rather than their age. Estimates of the age regression on lifetime conduct disorder symptoms in the community-based male adolescents were applied to the clinical sample. The resulting standardized residual scores were used in all subsequent analyses.
The Colorado Adolescent Rearing Inventory (26) , a 48-item face-to-face interview, was used to assess four domains of childhood maltreatment: neglect (15 items), verbal/psychological abuse (8 items), physical abuse (12 items), and sexual abuse (13 items). The Colorado Adolescent Rearing Inventory interview and instruction manual are available free of charge on the web (http://ibgwww.Colorado.edu/cadd/a_drug/links/cari_home.html). Details about the development and standardization of the inventory have been previously published (26) ; a significant relationship was also seen between Colorado Adolescent Rearing Inventory scores and DSM-IV conduct disorder symptom counts in both adolescent patients and matched community comparison adolescents.
The estimated internal consistency for the total Colorado Adolescent Rearing Inventory score (computed as the sum of four domain scores) in the current study was quite high (Cronbach’s alpha=0.87) and did not differ substantially from that of the subscale scores from the four domains, which ranged from 0.64 for the verbal/psychological abuse scale to 0.86 for the sexual abuse scale.
Any endorsement of the 48 core items was followed up by a series of “probe” questions addressing subject age at first occurrence, subject relationship to the perpetrator, the duration or frequency of abuse/neglect, resulting injuries, treatments received, and living situation at the time of the event. Positive endorsement of any one (or more) of 18 items that were considered to be flagrant abuse (e.g., “Did anyone ever intentionally burn you, for example, with cigarettes, or matches, or scalding water, or a stove top?”) required reporting to a child welfare agency.
For the present study, positive endorsement of each individual item was weighted by its corresponding duration (neglect) or frequency (abuse) level (obtained through the probe questions) in order to maximize the severity information contained in the scores. We computed the sum of the weighted domain scores to create a total Colorado Adolescent Rearing Inventory “severity” score that reflected both the breadth and depth of the maltreatment. The resulting scores were log-transformed to reduce skewness. The weighted Colorado Adolescent Rearing Inventory score correlates well (r=0.82, p<0.01) with the original, unweighted score previously studied (26) .
Social class was estimated from parental reports of educational and occupational status modified from the work of Hollingshead and Redlich.
DNA Extraction and Genotyping
Buccal cell DNA was extracted as described previously (23) . The 30-bp variable number tandem repeat polymorphism in the 5′ promoter region of the monoamine oxidase A (MAOA) gene was assayed as described by Sabol and Hamer (27) . PCR reactions contained 1 μL of DNA (20 ng or less), 1.8 mM MgCl 2 , 180 μΜ deoxynucleotides, with 7′-deaza-2′-deoxyGTP (Roche Applied Science, Indianapolis) substituted for one half of the dGTP, 200 nM forward and reverse primers (IDT, Coralville, Iowa) and 1 unit of AmpliTaq Gold® polymerase (PE-Biosystems, Foster City, Calif.), in a total volume of 20 μL. Primer sequences were forward, 5′-ACAGCCTGACCGTGGAGAAG-3′ (fluorescently labeled), and reverse, 5′-GAACGTGACGCTCCATTCGGA-3′. Amplification was performed using touchdown PCR (28) . A 95°C incubation for 10 minutes was followed by two cycles of 95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 60 seconds. The annealing temperature was decreased every two cycles from 65°C to 57°C in 2°C increments (10 cycles total), and a final 30 cycles of 95°C for 30 seconds, 65°C for 30 seconds, and 72°C for 60 seconds and a final 30-minute incubation at 72°C. One μL of PCR product was then combined with 2 μL of loading buffer containing size standard (Genescan 500 TAMRA® PE-Biosystems); PCR products were analyzed in an ABI Prism 377 ABI Genetic Analyzer using the protocols that were supplied by the company. Products of this reaction included three fragment sizes that ranged between 291 and 351 bp (2 to 4 repeats). In the current study group, the 3-repeat and 4-repeat alleles (321 bp and 351 bp) were the most common, with rates of 28% and 71%, respectively. On the basis of evidence suggesting that allelic groups can be pooled along the lines of their effects on transcriptional efficiency (27 , 29 , 30) , those with the 2-repeat allele (291 bp; 1%) were combined with those with the 3-repeat allele (321 bp; 28%). A sequence length of at least 351 bp (4 repeats) is optimal for high expression (27) . Thus, the shorter allele category (291 and 321) is functionally classified as the risk allele associated with low MAO-A activity. Because this MAO-A polymorphism is located on the X chromosome, the current male sample is hemizygous; the allele frequencies after pooling were 29% for the 321-bp allele and 71% for the 351-bp allele.
Biological parents and siblings of the patients were also genotyped, providing the data necessary to confirm Mendelian inheritance. This method of error checking, along with considerable numbers of random duplicate genotyping of individuals, maximizes our confidence in the accuracy of the allele calls. Incompatible genotypes were detected in less than 1% of the sample; in these cases individuals were excluded from all analyses. Table 1 shows the allele frequencies divided among our ethnic groups. Results of a chi-square analysis of proportions showed no significant differences in allele frequencies by ethnic group.
Results
Rates of Neglect and Abuse
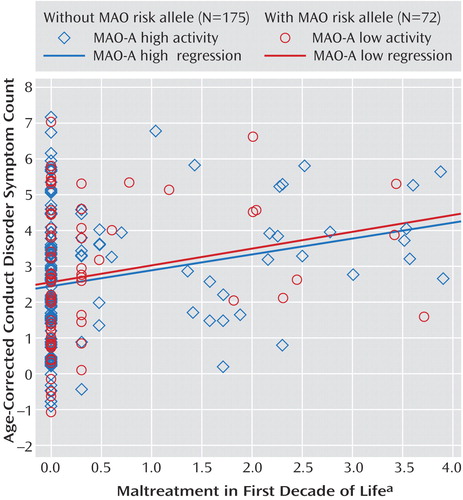
Neglect and abuse were not uncommon in this clinical cohort: nearly half of the adolescent boys reported at least one maltreatment event. Table 2 shows the endorsement rates of maltreatment occurring by age 11. This “first decade of life” criterion was applied in order to closely match the timeframe used in the Caspi et al. (14) study. More than one-third of the subjects reported some neglect (17.4%) or physical abuse (16.6%), while the prevalence of verbal and sexual abuse was slightly less pronounced.
The most frequently endorsed neglectful event was being repeatedly threatened with abandonment (7.3%). Patients frequently reported being yelled at daily (8.1%), being hit with a fist (9.3%), being given alcohol or drugs (9.7%), and being hit with a belt, whip, cord, etc. (14.6%). Table 2 also shows endorsement rates of maltreatment when age of first occurrence was ignored (i.e., occurrence in childhood or adolescence). Although rates were higher for all four domains, rates of sexual abuse were elevated most dramatically, increasing from 5.2% with one or more events to 19.0%. Endorsement rates for maltreatment of any form increased from 28.3% to 45.8% when comparing the first decade of life to all of childhood/adolescence; rates falling into the “severe” category more than doubled.
Conduct Disorder
All of the youths had been referred for serious conduct problems. According to information from the self-report DISC-IV interview, 87.1% of the patients met DSM criteria for conduct disorder. Although 12.9% (N=32) of the patients failed to meet criteria for conduct disorder, most (N=27) met criteria for one or two symptoms; only five boys (2.0% of the entire cohort) denied a history of any conduct disorder symptoms. Overall, the adolescent boys in the current study represent a severely behaviorally disturbed group. The mean number of symptoms in this patient cohort (mean=5.83, SD=2.88) is nearly twice the required number to meet criteria for a lifetime diagnosis.
Predicting Conduct Disorder Severity
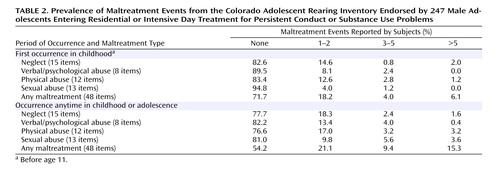
Standard regression analyses were conducted to test whether conduct disorder severity scores could be predicted by weighted maltreatment scores, MAO-A genotype, and the interaction between weighted scores and MAO-A. The MAO-A genotypes were dummy coded as 0 (low activity) and 1 (high activity). These analyses used only those neglect/abuse endorsements with reported age of first occurrence before age 11. Figure 1 displays a two-group scatterplot of the weighted maltreatment scores against conduct disorder scores. Regression lines were fit for each group and are displayed to facilitate group comparison.
a Positive endorsement of any individual maltreatment item from the Colorado Adolescent Rearing Inventory was weighted by its corresponding duration or frequency. Weighted scores for each maltreatment type were summed, resulting in overall severity ratings (none: 0, mild: <2, moderate: 2–5; severe: >5).
Consistent with the Caspi et al. findings (14) , the main effect for maltreatment on conduct problems was significant (standardized β=0.23; R 2 =0.05, p<0.01), but the main effect for genotype was not (β=0.03; R 2 <0.01, p=0.62). However, we observed no significant interaction between weighted maltreatment scores and MAO-A (β<0.01; R 2 <0.01, p=0.94). We also examined this model with each of the four maltreatment types. As shown in Figure 2 , no significant interactions with MAO-A genotype were detected for neglect (β=–0.06, p=0.39), verbal/psychological abuse (β=–0.06, p=0.45), physical abuse (β=0.07, p=0.32), or sexual abuse (β=0.05, p=0.45).
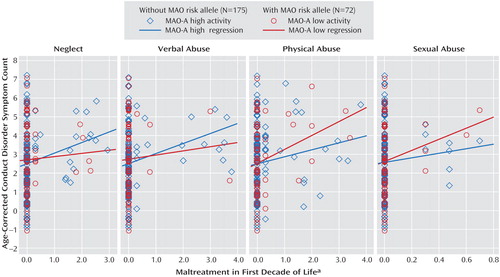
a Positive endorsement of any individual maltreatment item from the Colorado Adolescent Rearing Inventory was weighted by its corresponding duration or frequency. Weighted scores for each maltreatment type were summed, resulting in overall severity ratings (none: 0, mild: <2, moderate: 2–5; severe: >5).
We conducted secondary analyses of the MAO-A polymorphism in which weighted maltreatment scores included all neglect and abuse events occurring across childhood and adolescence (i.e., not restricted to “first decade of life”). The more cumulative scores correlated very strongly with the original early childhood scores ranging from r=0.68 for sexual abuse to r=0.99 for all other domains. Thus, we did not expect a meaningful change in the interaction analysis, which indeed yielded essentially identical results to the childhood-only analyses.
In addition, we included a measure of socioeconomic status, derived from parental education and occupation data, as a covariate in the MAO-A regression analyses. Socioeconomic status was very weakly associated with Colorado Adolescent Rearing Inventory scores (r=–0.01 to –0.02) as well as with conduct disorder (r=0.05), and had a negligible impact on the results of the MAO-A interaction analyses.
Estimated Power Through Simulation
To estimate the level of statistical power we had to detect an interaction effect in the regression models, a series of simulations were performed. Using the fixed main effect values from the Colorado Adolescent Rearing Inventory weighted analysis, we simulated 1,000 datasets of our current cohort size (N=247), varying the interaction effect from β=0.10 to β=0.40 in increments of 0.02. Results of the simulation demonstrated that we had 90% power to detect an effect of β=0.20 and 80% power to detect an effect of β=0.18, which is approximately half the effect size of that reported by Caspi et al. (14) (β=0.36).
Discussion
The Caspi et al. (14) study was the first to suggest a genotype-environmental interaction for a childhood psychiatric disorder. A polymorphism in the MAO-A gene was chosen for the study because it encodes for the MAO-A enzyme, which metabolizes norepinephrine, dopamine, and serotonin. Thus, a deficiency in the enzyme may lead to increased levels of these neurotransmitters. Numerous animal and human studies have linked low MAO-A activity to aggression, antisocial behavior, and increased responses to stress (31 – 36) . Although a direct effect of MAO-A activity on antisocial behavior was not demonstrated, Caspi and colleagues reported a significant interaction between childhood maltreatment and MAO-A genotype in predicting subsequent antisocial behavior. That is, individuals who reported high levels of maltreatment and who possessed the low MAO-A activity allele were more likely to develop antisocial behavior than those with low levels of maltreatment.
The Caspi et al. (14) report called for replication studies using more systematic assessment of childhood maltreatment and patient groups that could illuminate the clinical relevance of the result. The current study addressed both of these issues. We examined whether this genetic-environmental interaction could be detected in a group of clinically ascertained male adolescents who were entering treatment for significant conduct and substance use problems. Patients were thoroughly assessed with a detailed, validated interview (26) for a history of childhood neglect and physical and sexual abuse by parents, caregivers, or other adults. Age at first occurrence, frequency, and duration information was included in a composite measure of maltreatment in the first decade of life. Four domain scores (neglect, verbal/psychological abuse, physical abuse, and sexual abuse) were also used to assess the specificity of effects. As expected, maltreatment was significantly associated with severity of conduct disorder on the basis of DSM-IV lifetime symptoms. Similar to the findings in the Dunedin sample, our adolescent patients with the low-activity MAO-A allele did not differ from those with the high-activity allele in their levels of conduct disorder. However, results of the interaction analyses in our patient group did not confirm the Caspi et al. (14) findings. There were no significant differences in the relationship between maltreatment and conduct disorder in the two MAO-A genotype groups for the overall Colorado Adolescent Rearing Inventory score or the four domain scores. A follow-up analysis, which utilized a measure of neglect and abuse events through middle childhood and adolescence, showed nearly identical results. We note that this nonreplication was demonstrated in a cohort with 80% power to detect an effect size that was approximately half the magnitude reported in the Dunedin study.
The present study design has a number of strengths. Because the sample was clinically ascertained, we observed a great deal of variation in levels of conduct disorder as well as severity of childhood maltreatment. Although the assessments relied on retrospective report, many of the target behaviors/events had occurred in the very recent past and may be ongoing. Unlike some previous work, neglect was carefully assessed as part of the abuse/neglect syndrome. Additionally, DNA samples were collected from the siblings and parents of the male patients. Thus, MAO-A genotype data could be verified with more confidence than is possible with singleton samples.
Although there are many advantages to our approach, there are limitations as well. Nearly all of the patients had some history of conduct problems, and over half had experienced some form of maltreatment. Unlike random population samples, the ascertainment of clinical cohorts can impose an attenuation of the true correlation among variables that are part of the selection process. Thus, we may be underestimating the correlation between maltreatment and conduct disorder severity. However, the allele frequencies in this clinical cohort did not differ substantially from those in the Dunedin study (32.2% versus 33.9% for the low activity allele in the Colorado [Caucasian] and Dunedin subjects, respectively).
Second, the Dunedin study obtained data on antisocial behavior in young adulthood, which was utilized in their composite outcome variable. The current subjects have just begun to age into young adulthood when patterns of adult criminal behavior and psychopathy may emerge. We are conducting a follow-up study of these patients to investigate the predictors of persistent antisocial behavior, and it will be important to examine whether MAO-A activity has a role in the risk for adult antisocial personality disorder. Conduct disorder severity scores were also based on self-report interviews.
Conclusions
Aggression, irresponsibility, and reckless disregard for the safety of others, which are among the diagnostic criteria for antisocial personality disorder, can certainly be expressed as neglect or abuse of children. On the basis of direct parental interviews, we estimate that 76% of the probands had at least one parent who endorsed one or more DSM-IV criteria for antisocial personality disorder. This observation, along with results from a number of studies involving genetically informative samples (see Rhee and Waldman [37] for a review and meta-analysis), strongly suggests that the familial aggregation of antisocial behavior is largely due to genetic factors. Thus, we must consider the possibility that in addition to any environmental risk it may pose, maltreatment may also be a behavioral expression of the genes shared by parents and their children.
1. Loeber R, Burke JD, Lahey BB: What are adolescent antecedents to antisocial personality disorder? Crim Behav Ment Health 2002; 12:24–36Google Scholar
2. Ridenour TA, Cottler LB, Robins LN, Compton WM, Spitznagel EL, Cunningham-Williams RM: Test of the plausibility of adolescent substance use playing a causal role in developing adulthood antisocial behavior. J Abnorm Psychol 2002; 111:144–155Google Scholar
3. Robins LN, Price RK: Adult disorders predicted by childhood conduct problems: results from the NIMH Epidemiological Catchment Area Project. Psychiatry 1991; 54:116–132Google Scholar
4. Lahey BB, Waldman ID, McBurnett K: Annotation: the development of antisocial behavior: an integrative causal model. J Child Psychol Psychiatry 1999; 40:669–682Google Scholar
5. Loeber R: Antisocial behavior: more enduring than changeable? J Am Acad Child Adolesc Psychiatry 1991; 30:393–397Google Scholar
6. Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M: Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J Abnorm Psychol 2002; 11:411–424Google Scholar
7. Pedersen W, Mastekaasa A, Wichstrom L: Conduct problems and early cannabis initiation: a longitudinal study of gender differences. Addiction 2001; 96:415–431Google Scholar
8. Armstrong TD, Costello EJ: Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol 2002; 70:1224–1239Google Scholar
9. Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK: Genetic and environmental influences on behavioral disinhibition. Am J Med Genet 2000; 96:684–695Google Scholar
10. Dierker LC, Merikangas KR, Szatmari P: Influence of parental concordance for psychiatric disorders on psychopathology in offspring. J Am Acad Child Adolesc Psychiatry 1999; 38:280–288Google Scholar
11. Kuperman S, Schlosser SS, Lidral J, Reich W: Relationship of child psychopathology to parental alcoholism and antisocial personality disorder. J Am Acad Child Adolesc Psychiatry 1999; 38:686–692Google Scholar
12. Marmorstein NR, Iacono WG: Major depression and conduct disorder in youth: associations with parental psychopathology and parent-child conflict. J Child Psychol Psychiatry 2004; 45:377–386Google Scholar
13. Rutter M, Giller H, Hagell A: Antisocial Behavior by Young People. New York, Cambridge University Press, 1998Google Scholar
14. Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IQ, Taylor A, Poulton R: Role of genotype in the cycle of violence in maltreated children. Science 2002; 297:851–854Google Scholar
15. Silva PA, Stanton W (eds): From Child to Adult: The Dunedin Study. Oxford, Oxford University Press, 1996Google Scholar
16. Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Muller U, Aguet M, Babinet C, Shih JC: Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science 1995; 168:1763–1766Google Scholar
17. Olivier B, Mos J: Rodent models of aggressive behavior and serotonergic drugs. Prog Neuropsychopharmacol Biol Psychiatry 1992; 16:847–870Google Scholar
18. Rodriguiz RM, Chu R, Caron MG, Wetsel WC: Aberrant responses in social interaction of dopamine transporter knockout mice. Behav Brain Res 2004; 148:185–198Google Scholar
19. Sanchez C, Arnt J, Hyttel J, Moltzen EK: The role of serotonergic mechanisms in inhibition of isolation-induced aggression in male mice. Psychopharmacology (Berl) 1993; 110:53–59Google Scholar
20. Halperin JM, Schulz KP, McKay KE, Sharma V, Newcorn JH: Familial correlates of central serotonin function in children with disruptive behavior disorders. Psychiatry Res 2003; 11:205–216Google Scholar
21. Krakowski M: Violence and serotonin: influence of impulse control, affect regulation, and social functioning. J Neuropsychiatry Clin Neurosci 2003; 15:294–305Google Scholar
22. Miczek KA, Fish EW, De Bold JF, De Almeida RM: Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and gamma-aminobutyric acid systems. Psychopharmacology (Berl) 2002; 163:434–458Google Scholar
23. Stallings MC, Corley RC, Hewitt JK, Krauter KS, Lessem JL, Mikulich SK, Rhee SH, Smolen A, Young SE, Crowley TJ: A genome-wide search for quantitative trait loci influencing substance dependence vulnerability in adolescence. Drug Alc Depend 2003; 70:295–307Google Scholar
24. Stallings MC, Corley RC, Hewitt JK, Krauter KS, Lessem JL, Mikulich SK, Rhee SH, Smolen A, Young SE, Crowley TJ: A genome-wide search for quantitative trait loci influencing antisocial drug dependence in adolescence. Arch Gen Psychiatry 2005; 62:1042–1051Google Scholar
25. Young SE, Corley RP, Stallings MC, Rhee SH, Crowley TJ, Hewitt JK: Substance use, abuse and dependence in adolescence: prevalence, symptom profiles and correlates. Drug Alc Depend 2002; 68:309–322Google Scholar
26. Crowley TJ, Mikulich SK, Ehlers KM, Hall SK, Whitmore EA: Discriminative validity and clinical utility of an abuse-neglect interview for adolescents with conduct and substance use problems. Am J Psychiatry 2002; 160:1461–1469Google Scholar
27. Sabol SZ, Hamer D: A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet 1998; 103:273–279Google Scholar
28. Don RH, Cox PT, Wainwright BJ, Baker K, Mattick JS: “Touchdown” PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res 1992; 19:4008Google Scholar
29. Deckert J, Catalano M, Syagailo YV, Bosi M, Okladnova O, Di Bella D, Nothen MM, Maffei P, Franke P, Fritze J, Maier W, Propping P, Beckmann H, Bellodi L, Lesch K: Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Hum Mol Genet 1999; 8:621–624Google Scholar
30. Denney RM, Koch H, Craig IW: Association between monoamine oxidase A activity in human male skin fibroblasts and genotype of the MAOA promoter-associated variable number tandem repeat. Hum Genet 1999; 105:542–551Google Scholar
31. Brunner HG: MAOA deficiency and abnormal behaviour: perspectives on an association. Ciba Found Symp 1996; 194:155–164Google Scholar
32. Lesch KP, Merschdorf U: Impulsivity, aggression and serotonin: a molecular psychobiological perspective. Behav Sci Law 2000; 18:581–604Google Scholar
33. Rowe DC: Biology and Crime. Los Angeles, Roxbury, 2001Google Scholar
34. Shih JC, Chen K, Ridd MJ: Monoamine oxidase: from genes to behavior. Ann Rev Neurosci 1999; 22:197–217Google Scholar
35. Shih JC: Cloning, after cloning, knock-out mice, and physiological functions of MAO A and B. NeuroToxicology 2004; 25:21–30Google Scholar
36. Virkkunen M, Linnoila M: Serotonin in early-onset alcoholism. Recent Dev Alcohol 1997; 13:173–189Google Scholar
37. Rhee SH, Waldman ID: Genetic and environmental influences on antisocial behavior: a meta-analysis of twin and adoption studies. Psych Bull 2002; 128:490–529Google Scholar