An Inverse Relationship Between Perceived Harm and Participation Willingness in Schizophrenia Research Protocols
Abstract
Objective: This study attempted to clarify how people with schizophrenia evaluate the potential harm associated with various research-related procedures and how these assessments relate to participation willingness. Method: The authors conducted a semistructured interview among participants with schizophrenia. Results: Sixty participants with schizophrenia rated four procedures as harmful (e.g., symptom induction), five procedures as moderately harmful (e.g., being given a placebo), and six procedures as not harmful (e.g., undergoing a physical examination). Rated willingness to participate was inversely related to the participants’ perceptions of harmfulness for all procedures. Conclusions: In this study, people living with schizophrenia perceived different research procedures as posing different levels of possible harm. Potential harm appears to be an important consideration in protocol enrollment decisions. This work reaffirms the value of clarifying the strengths of seriously ill people who may choose to participate in research.
The serious nature of schizophrenia creates a motivating force for pursuing clinical research to understand this devastating neuropsychiatric disease affecting 1% of the world’s population. However, the same features of the illness that inspire us to study its origins and treatment also give rise to ethical challenges. As protocol volunteers, people with schizophrenia have potential sources of vulnerability because cognitive impairments, symptoms, and other factors may interfere with informed consent for research participation (1 – 2) . Regulatory and ethical safeguards for the protection of human subjects have been designed to facilitate nonexploitative studies on potentially vulnerable persons (3 – 5) . Nevertheless, controversy still surrounds key ethical issues in schizophrenia research, including whether people living with serious mental illnesses are able to discern harm associated with protocol participation (2 , 5) .
Data are necessary to provide a scientific foundation for ethical safeguards in psychiatric research. Efforts to improve the informed consent process could be enhanced by knowing whether people with schizophrenia logically assess the risks of protocols and make decisions about participation that take those assessments into account (6 – 8) . Few studies have explored these issues. More than two decades ago, Stanley et al. (9) found that psychiatric and medical hospital patients were generally similar in their willingness to participate in research studies with varying levels of risk and benefit. More recently, our group found that individuals with schizophrenia expressed less willingness to participate in trials perceived as posing a higher risk (8 , 10) . The current study was designed to extend the previous findings by examining how people with schizophrenia view research involving specific research procedures.
Method
For this study (funded by the National Institute of Mental Health and approved by the University of New Mexico Health Sciences Center’s institutional review board), we developed a questionnaire with 298 quantitative scaled questions and six qualitative items exploring ethically important considerations in mental illness research. In one section, the participants were asked to rate their willingness to participate in projects involving each of 15 procedures and their views of harm associated with the procedures. We also administered several standard scales, e.g., the Brief Symptom Inventory (11) .
People with schizophrenia were recruited by community outreach or physician referral. Diagnosis was confirmed by chart reviews with participant consent. A trained interviewer administered the survey by reading each question and recording responses. Most participants completed the survey in 2.5 to 3 hours. All participants received $30 for their time. Data were confidentially encoded.
Results
Sixty individuals with schizophrenia volunteered. Most were men (80%) and unmarried (90%), with a mean age of 44.3 years (SD=10.7). The majority (60%) were white, with 22% of Hispanic origin. Almost half (42%) had only a high school education, half (54%) had some college education, and a few (5%) had postgraduate education.
Brief Symptom Inventory scale scores ranged from 0 to 3.8, with mean=1.4 (SD=0.9) for global severity, mean=32.1 (SD=14.3) for positive symptom total, and mean=2.1 (SD=0.7) for positive symptom distress index. All participants had adequate reading and auditory comprehension for our project, as measured by the Woodcock Reading Mastery Test (12) and the Boston Diagnostic Aphasia Examination (13) .
Perceived harm versus willingness to participate is shown in Figure 1 . The participants rated harmfulness on a scale (1=not harmful at all to 5=very harmful) for 15 evaluation or intervention procedures. The participants rated projects involving taking a dangerous new experimental medication, experiencing temporary symptoms of schizophrenia, having a spinal tap, or discontinuing their usual medication for 2 weeks as harmful (mean=3.70–4.01, SD=1.45–1.83). The participants rated projects involving taking a new experimental medication, viewing an upsetting word or picture, taking a placebo, taking a different medication while in the hospital, or discontinuing their usual medication for 2 days as moderately harmful (mean=2.74–3.34, SD=1.59–2.04). They viewed projects involving an X-ray of the head, an X-ray of the body, having a physical examination, filling out questionnaires, talking with a researcher, or having their blood pressure taken as not very harmful (mean=1.23–2.22, SD=0.83–1.62). This analysis produced a main effect of procedure (F=26.26, df=14, 45, p<0.0001; pooled SD=1.56, maximum Cohen’s d across procedures=1.81).
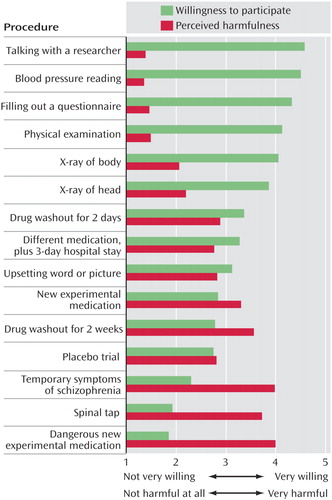
a Correlation of rated harm and willingness to participate and harmfulness ranged from –0.26 to –0.75, with r=0.26, p<0.05 for filling out the questionnaire and r=–0.45 to –0.75, mean r=–0.58, all p<0.001 for all other procedures.
The participants rated their willingness to participate (1=not very willing to 5=very willing) in research projects involving each of the 15 evaluation procedures. A procedure main effect was found (F=17.46, df=14, 45, p<0.0001; pooled SD=1.79, maximum Cohen’s d across procedures=1.56).
The participants were very willing to participate in projects involving talking with a researcher, having their blood pressure taken, filling out questionnaires, having a physical examination, or having an X-ray of the body or an X-ray of the head (mean=3.92–4.65, SD=1.13–1.73). They were moderately willing to participate in projects involving stopping their usual medication for 2 days, taking a different medication while in the hospital, viewing an upsetting word or picture (i.e., for symptom provocation), taking a new experimental medication, stopping their usual medication for 2 weeks, or taking a placebo (mean=2.61–3.48, SD=1.98–2.12). They were not very willing to participate in projects involving experiencing temporary symptoms of schizophrenia, having a spinal tap, or taking a dangerous new experimental medications (mean=1.84–2.12, SD=1.61–1.91). Rated willingness to participate was inversely related to rated harmfulness for all procedures (r=–0.26, p<0.05, for filling out questionnaires; r=–0.45 to –0.75, mean r=–0.58, all p<0.001 for the other 14 procedures). There was no consistent pattern of correlation between willingness to participate and age, education, or scores on standardized scales.
Discussion
The people with schizophrenia in this study perceived the potential harms associated with evaluative and intervention research procedures in a manner that was both logical and clear. Furthermore, they indicated that their willingness to enroll in projects was diminished for those that involved greater perceived harm. These findings confirm results of prior studies and may offer reassurance about the ability of some people with schizophrenia to make reasoned decisions about research participation. In addition to demonstrating the discernment of research participants with schizophrenia, this study provides data on volunteers’ opinions about specific research procedures. Defining acceptable levels of risk in research with seriously ill groups with multiple potential sources of vulnerability can be difficult, and the views of potential research participants may facilitate attempts to attain consensus about procedures that have remained controversial within the scientific community.
Our findings may also alert investigators and members of institutional review boards to the possibility of discrepancies between physicians’ and participants’ perceptions of the harmfulness of certain procedures. In this study, the participants ranked protocols that involved viewing an upsetting word or picture (i.e., in the symptom-provocation study) as about as harmful as a 2-day medication washout study, a 3-day hospital stay with a different medication, or a placebo trial. More research is needed to determine whether individuals without serious mental illness would consider viewing upsetting visual stimuli equally risky or whether this research technique is particularly disturbing to people with schizophrenia. Such procedures may merit enhanced educational efforts during the process of informed consent (2 , 9) .
The limitations of this study include its reliance on self-reported data rather than on actual decisions or behaviors associated with research participation and the lack of a comparison group. Our study volunteers were predominantly middle aged and men, with Brief Symptom Inventory scores indicating significant symptoms. The strength of this study lies in its novel attempt to bring participants’ perspectives into the ethical discussion of the risks of various procedures in clinical protocols. We hope that this study inspires more in-depth examination of the views of our respected partners in research.
1. Roberts LW, Roberts B: Psychiatric research ethics: an overview of evolving guidelines and current ethical dilemmas in the study of mental illness. Biol Psychiatry 1999; 46:1025–1038Google Scholar
2. Carpenter WT Jr, Gold JM, Lahti AC, Queern CA, Conley RR, Bartko JJ, Kovnick J, Appelbaum PS: Decisional capacity for informed consent in schizophrenia research. Arch Gen Psychiatry 2000; 57:533–538Google Scholar
3. National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research: The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. Washington, DC, US Government Printing Office, 1979Google Scholar
4. Dresser R: Mentally disabled research subjects: the enduring policy issues. JAMA 1996; 276:67–72Google Scholar
5. Appelbaum PS: Rethinking the conduct of psychiatric research. Arch Gen Psychiatry 1997; 54:117–120Google Scholar
6. Stanley B, Sieber JE, Melton GB: Empirical studies of ethical issues in research: a research agenda. Am Psychol 1987; 42:735–741Google Scholar
7. Dunn LB, Jeste DV: Enhancing informed consent for research and treatment. Neuropsychopharmacology 2001; 24:595–607Google Scholar
8. Roberts LW, Warner TD, Nguyen KP, Geppert CM, Rogers MK, Roberts BB: Schizophrenia patients’ and psychiatrists’ perspectives on ethical aspects of symptom re-emergence during psychopharmacological research participation. Psychopharmacology (Berl) 2003; 171:58–67Google Scholar
9. Stanley B, Stanley M, Lautin A, Kane J, Schwartz N: Preliminary findings on psychiatric patients as research participants: a population at risk? Am J Psychiatry 1981; 138:669–671Google Scholar
10. Roberts LW, Warner TD, Brody JL, Roberts B, Lauriello J, Lyketsos C: Patient and psychiatrist ratings of hypothetical schizophrenia research protocols: assessment of harm potential and factors influencing participation decisions. Am J Psychiatry 2002; 159:573–584Google Scholar
11. Derogatis LR: Brief Symptom Inventory Administration, Scoring, and Procedures Manual. Minneapolis, NCS Pearson, 1993Google Scholar
12. Woodcock RW: Woodcock Reading Master Tests—Revised: Examiner’s Manual. Circle Pines, Minn, American Guidance Service, 1987Google Scholar
13. Goodglass H, Kaplan E: The Assessment of Phasia and Related Disorders, Second Edition. Philadelphia, Lea & Feibiger, 1983Google Scholar



