Dopamine Receptor Response to NMDA Stimulation
Dopamine D 1 receptors are evenly distributed in the plasma membrane of the direct pathway striatonigral neurons at rest, shown above by tagging D 1 receptors with a fluorescent marker and examining their expression in striatal neurons in culture ( Figure ). This technique shows D 1 receptor localization in cell body and plasma membrane of neurons but not in glia. Because D 1 receptors and the NMDA-sensitive glutamate receptor are known to interact functionally in the medium spiny neurons of the striatum, we studied the mechanism of this interaction. Stimulation of NMDA receptors with a direct acting agonist did, even in the presence of an NMDA-gated channel blocker, increase D 1 receptor-positive spines in the cells. In cell bodies, the proportion of D 1 receptors in the membrane relative to D 1 receptors in the cytosol (membrane/cytosol D 1 receptor ratio) significantly increased with NMDA receptor stimulation, but D 2 receptor localization was unaffected. At rest, a large fraction of the D 1 receptors freely diffuses within the plasma membrane. In the spines of dendrites, where both the NMDA and D 1 receptors can be associated with the postsynaptic density, the diffusion coefficient of the D 1 receptors is lower than in dendritic membrane. We were able to demonstrate that the reduced mobility of the D 1 receptors is the result of a direct interaction between D 1 and NMDA receptors that are allosterically transformed by occupation of the NMDA binding site. Specifically, the terminal end of D 1 receptors directly binds to the NR 1 subunit of the ligand-occupied NMDA receptor, effectively trapping D 1 receptors at the postsynaptic density when the NMDA receptors are stimulated. Notably, the binding of D 1 receptors to NR 1 is regulated by the occupation of the NMDA binding site but not by activation of the co-agonist site—the glycine receptor—nor by ion flow through the open NMDA ionophore. In summary, excitatory stimulation of the NMDA receptors will trap the D 1 receptors at the postsynaptic density, presumably making them stably available for dopaminergic stimulation and increasing the sensitivity of the postsynaptic density to dopamine itself. Conversely, this interaction could account for reduced D 1 receptor sensitivity in conditions characterized by reduced NMDA receptor activation. The allosteric modification of the ligand-occupied NMDA receptor, which can trigger the recruitment of D 1 receptors, but not D 2 receptors, to neuronal spines, represents a molecular mechanism for brain plasticity that could have an impact on cerebral development, function, and diverse psychiatric symptoms.
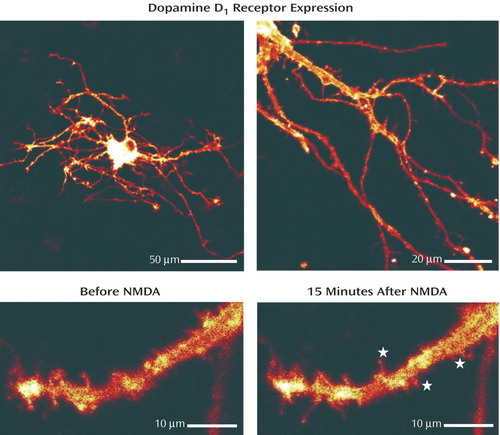
Figure 1. The top two images show D 1 receptor fluorescence tagging of a neuron in striatal culture (left) and extended focus projection from several confocal sections of a neuron (right). The bottom images show magnified segments of a dendritic tree, with stars indicating new D 1 receptor-positive spines after NMDA treatment. Image adapted from Scott L, Zelenin S, Malmersjo S, Kowalewski JM, Markus EZ, Nairn AC, Greengard P, Brismar H, Aperia A: Allosteric changes of the NMDA receptor trap diffusible dopamine 1 receptors in spines. Proc Natl Acad Sci U S A 2006; 103:762–767.



