Prediction of Level of Serotonin 2A Receptor Binding by Serotonin Receptor 2A Genetic Variation in Postmortem Brain Samples From Subjects Who Did or Did Not Commit Suicide
Abstract
OBJECTIVE: Postmortem studies have indicated that suicide victims have greater serotonin receptor 2A (5-HTR2A) binding in prefrontal brain regions. However, there remains some controversy regarding the biological specificity of these findings. The authors hypothesized that the variance observed in brain 5-HTR2A binding is genetically mediated, at least in part. METHOD: Postmortem data from 56 subjects who had committed suicide and 126 normal comparison subjects were studied; brain tissue was available from 11 subjects who committed suicide and 11 comparison subjects. Homogenate binding assays were carried out with [3H]ketanserin. Variation at the 5-HTR2A gene (HTR2A) was investigated by means of two polymorphisms: T102C and A-1438G. RESULTS: 5-HTR2A binding was greater in the prefrontal cortex of the subjects who committed suicide. In addition, the findings suggest that HTR2A variation significantly affects 5-HTR2A binding. However, no interaction between suicidal behavior and this locus was observed. CONCLUSIONS: These results confirm previous reports of greater 5-HTR2A binding in subjects who committed suicide; they also provide preliminary evidence suggesting that the number of 5-HTR2A receptors is genetically mediated.
Suicide is a serious public health problem that ranks among the 10 most frequent causes of death. CSF, urine, platelet, and neuroendocrine challenge test studies have indicated that alterations in the serotonin system may play an important role in suicidal behavior (reviewed by Mann [1]). Consistent with these findings, a number of postmortem studies have reported that suicide victims have greater serotonin receptor 2A (5-HTR2A) binding in the prefrontal cortex than normal comparison subjects (2–5). It is thought that these findings may result from mechanisms such as adaptive or compensatory 5-HTR2A up-regulation secondary to reduced serotonergic neurotransmission or genetic mediation (6). However, there remains some controversy because these alterations could result from confounding factors such as psychotropic drug exposure.
In this article we report preliminary evidence, obtained in postmortem brain samples of suicide victims and matched comparison subjects, suggesting that the gene that codes for 5-HTR2A (HTR2A) has a role in the determination of the number of 5-HT2A receptors in the prefrontal cortex.
METHOD
Subjects were recruited as part of an ongoing collaboration with the Coroner’s Office of the Montreal Central Morgue. In this study, postmortem data for 56 subjects who had committed suicide and 126 normal comparison subjects were included. Brain tissue from the convex part of the superior frontal cortex (Brodmann’s area 8/9) was available for 5-HTR2A binding studies from 11 subjects who committed suicide and 11 comparison subjects. All suicide victims and comparison subjects were of similar ethnic origin. Moreover, suicide victims and comparison subjects for whom postmortem brain samples were obtained were matched for age, sex, and postmortem delay. Most of the comparison subjects for whom postmortem brain samples were obtained (N=7) were young victims of cardiovascular diseases. The remaining comparison subjects died in work-related accidents or in nonimpulsive road accidents. Written consents were signed by the families of all subjects.
Genomic DNA was extracted either from blood or frozen brain tissue by using standard procedures. We tested the HTR2A T102C and HTR2A A-1438G polymorphisms, which are, respectively, a silent mutation and a promoter variant with no proven functional significance. In addition, we tested an HTR2C polymorphism resulting from a G to C substitution that changes cystine to serine at position 23 of the serotonin 2C receptor. Polymerase chain reaction (PCR) was carried out in a total volume of 12.5 µl containing 40 ng of genomic DNA; 125 ng of the specific primers; 200 µM each of dGTP, dCTP, and dTTP; 25 µM dATP; 0.5 units of Taq DNA polymerase (Bio/Can Scientific, Mississauga, Ont., Canada); and 2.0 µl of 10X buffer (Bio/Can Scientific), with MgCl2 included in the final concentration of 1.5 mM. Samples were processed throughout 35 cycles of denaturation at 94°C, annealing specific to each primer pair, and elongation at 72°C. PCR products were digested with MspI (HTR2A T102C), Hpa II (HTR2A A-1438G), and HinfI (HTR2C) at 37°C for 1 hour and analyzed on a 2% agarose gel. Gels were read and interpreted independently by two different readers blind to whether they were from suicide victims or comparison subjects; results from the two readers were identical.
Brains were collected at autopsy with a maximum postmortem delay of 32 hours. They were immediately transported on ice to the laboratory, where they were dissected according to standard procedures and kept at –80°C. Homogenate binding assays were carried out with [3H]ketanserin (specific activity=80.9 Ci/mmol, NEN Life Science Products, Boston) and were performed according to well-established procedures (7). All experiments were carried out in duplicate (two tubes for total binding and two tubes for nonspecific binding). Specific binding was defined as the total binding minus the nonspecific counts obtained in the presence of 1 µM methysergide.
Difference in allele and haplotype distributions were analyzed by chi-square tests and odds ratios. A two-way analysis of variance was carried out to investigate the effect of having committed suicide or not and HTR2A on 5-HT2 binding. Subsequently, a logistic regression was conducted with suicide as the main outcome. All statistical analyses were carried out by using programs 4F, 4V, 7D, and LR of the BMDP statistical package.
RESULTS
The mean age of suicide victims at death was 36.09 years (SD=10.75); the mean age of comparison subjects at death was 33.54 years (SD=9.95). Twenty-six (46.4%) of the suicide victims died by hanging, eight (14.3%) by carbon monoxide poisoning, six (10.7%) by shooting, five (8.9%) by drug overdose, and the remaining 11 (19.6%) by different methods. Mean postmortem delay for the 11 suicide victims whose brain tissue was available was 23 hours (SD=5); the delay for the 11 comparison subjects was 26 hours (SD=4).
No difference was observed in radioligand affinity between groups (t=0.75, df=20, p=0.46), indicating that differences in ligand binding (Bmax) reflect differences in receptor number. For suicide victims, Bmax=2.78 fmol/mg (SD=0.88); for comparison subjects, Bmax=2.07 fmol/mg (SD=0.53). A two-way analysis of variance showed a significant main effect of being a suicide victim or not on 5-HTR2A binding (figure 1). This analysis also revealed that HTR2A variation affected 5-HTR2A binding (figure 1). However, as seen in figure 1, this locus affected binding equally in both groups (interaction F=0.21, df=1, 40, p>0.10). Accordingly, a stepwise logistic regression conducted with suicide as the main outcome indicated that 5-HTR2A binding, but not HTR2A variation (beta=1.11, SE=0.77, p=0.13), was statistically significant in the prediction of suicide (beta=1.43, SE=0.52, p=0.002). Consistent with the latter finding, no difference was observed in allele or haplotype distribution between groups when all subjects were tested (odds ratio=0.78, 95% confidence interval [CI]=0.48–1.28). Results observed for HTR2C were not significant (odds ratio=0.35, 95% CI=0.12–1.05).
DISCUSSION
These results provide preliminary evidence suggesting that 5-HTR2A binding is in part genetically determined by variation at the gene coding for 5-HTR2A. The relatively large effect size observed for suicide victims with haplotype 102T/-1438A compared with nonsuicide subjects with haplotype 102C/-1438G (effect size=2.18) was unexpected. Among other possible factors, this could be caused by an overrepresentation in our study group of subjects who committed violent suicide. Indeed, five (8.9%) of the 56 suicide victims and only one of the 11 included in the brain study used methods considered nonviolent. This is important because it has been reported that victims of violent suicide are more likely to demonstrate serotonergic abnormalities (8–11).
The T102C marker investigated is a silent T-to-C base substitution (12). In addition, preliminary studies using cell assays indicate that both variants of the –A1438G polymorphism present similar basal promoter activity (13), suggesting that this polymorphism is also nonfunctional. Thus, at this point, the most plausible interpretation is that both variants are in linkage disequilibrium with another polymorphism in this gene that may have a functional role.
Finally, our results do not support the hypothesis that HTR2A variation has a major role in the predisposition to suicide. Although one could speculate about possible explanations for why genotype could affect binding but not behavior, at this point we cannot reliably exclude a type II error accounting for the negative association. It may be quite possible that HTR2A does have a role in suicide susceptibility, but the number of subjects in this study did not afford enough power to detect this effect. Therefore, both positive and negative findings should be regarded as preliminary and in need of replication. We are in the process of collecting brains from additional suicide victims and comparison subjects to attempt to replicate these results in a larger study group. Similarly, it would be interesting to reproduce these findings in an independent sample of similar composition.
Received Nov. 13, 1998; revision received March 24, 1999; accepted March 26, 1999. From the Centre for Research in Neuroscience, Montreal General Hospital, McGill University; the Centre de Recherche Fernand Seguin, Hôpital L-H Lafontaine, Université de Montréal; the Département de Psychoéducation, Université du Québec à Hull; the Affective Disorders Unit, Royal Ottawa Hospital, University of Ottawa; and the Neurobiological Psychiatry Unit, Department of Psychiatry, McGill University. Address reprint requests to Dr. Turecki, Douglas Hospital Research Center, McGill University, 6875 LaSalle Blvd., Verdun, QC H4H 1R3, Canada; [email protected] (e-mail)
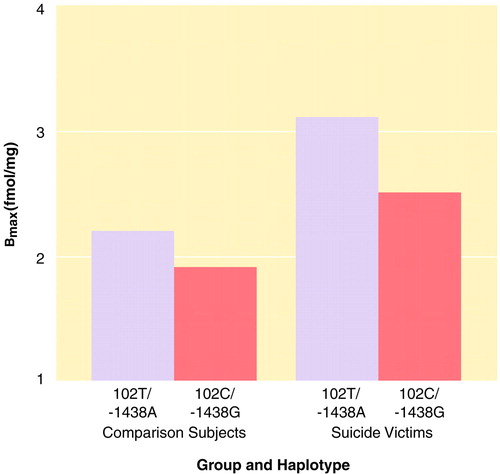
FIGURE 1. Distribution of 5-HTR2A Binding Sites (Bmax) in the Prefrontal Cortex of 11 Suicide Victims and 11 Normal Comparison Subjects and Two Polymorphisms of the HTR2A Gene (HTR2A)a
Two-way analysis of variance showed a significant main effect on 5-HTR2A binding of being a suicide victim or not (F=12.57, df=1, 40, p<0.01) and an effect of HTR2A variation (F=3.77, df=1, 40, p=0.05).
1. Mann JJ: The neurobiology of suicide. Nat Med 1998; 4:25–30Crossref, Medline, Google Scholar
2. Stanley M, Mann JJ: Increased serotonin-2 binding sites in frontal cortex of suicide victims. Lancet 1983; 1:214–216Crossref, Medline, Google Scholar
3. Mann JJ, Stanley M, McBride PA, McEwen BS: Increased serotonin2 and beta-adrenergic receptor binding in the frontal cortices of suicide victims. Arch Gen Psychiatry 1986; 43:954–959Crossref, Medline, Google Scholar
4. Arango V, Ernsberger P, Marzuk PM, Chen JS, Tierney H, Stanley M, Reis DJ, Mann JJ: Autoradiographic demonstration of increased serotonin 5-HT2 and beta-adrenergic receptor binding sites in the brain of suicide victims. Arch Gen Psychiatry 1990; 47:1038–1047Google Scholar
5. Hrdina PD, Demeter E, Vu TB, Sotonyi P, Palkovits M:5-HT uptake sites and 5-HT2 receptors in brain of antidepressant-free suicide victims/depressives: increase in 5-HT2 sites in cortex and amygdala. Brain Res 1993; 614:37–44Google Scholar
6. Mann JJ, Arango V, Underwood MD: Serotonin and suicidal behavior. Ann NY Acad Sci 1990; 600:476–484Crossref, Medline, Google Scholar
7. Hoyer D, Vos P, Closse A, Palacios JM, Engel G, Davies H: [3H]Ketanserin labels serotonin 5-HT2 and alpha 1-adrenergic receptors in human brain cortex. J Cardiovasc Pharmacol 1987; 10(suppl 3):S48–S50Google Scholar
8. Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK: Low cerebrospinal fluid 5-hydroxyindolacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci 1983; 33:2609–2614Google Scholar
9. Traskman-Bendz L, Alling C, Alsen M, Regnell G, Simonsson P, Ohman R: The role of monoamines in suicidal behavior. Acta Psychiatr Scand 1993; 371:45–47Crossref, Google Scholar
10. Nielsen DA, Goldman D, Virkkunen M, Tokola R, Rawlings R, Linnoila M: Suicidality and 5-hydroxyindoleacetic acid concentration associated with a tryptophan hydroxylase polymorphism. Arch Gen Psychiatry 1994; 51:34–38Crossref, Medline, Google Scholar
11. Nielsen DA, Virkkunen M, Lappalainen J, Eggert M, Brown GL, Long JC, Goldman D, Linnoila M: A tryptophan hydroxylase gene marker for suicidality and alcoholism. Arch Gen Psychiatry 1998; 55:593–602Crossref, Medline, Google Scholar
12. Warren JT Jr, Peacock ML, Rodriguez LC, Fink JK: An MspI polymorphism in the hyman serotonin receptor gene (HTR2): detection by DGGE and RFLP analysis. Hum Mol Genet 1993; 2:338Crossref, Medline, Google Scholar
13. Spurlock G, Heils A, Holmans P, Williams J, D’Souza UM, Cardno A, Murphy KC, Jones L, Buckland PR, McGuffin P, Lesch KP, Owen MJ: A family based association study of T102C polymorphism in 5HT2A and schizophrenia plus identification of new polymorphisms in the promoter. Mol Psychiatry 1998; 3:42–49Crossref, Medline, Google Scholar



