Neuropsychological Deficits in Adolescent-Onset Schizophrenia Compared With Attention Deficit Hyperactivity Disorder
Abstract
OBJECTIVE: Impaired neuropsychological performance involving abstraction-flexibility, memory, motor function, and attention has frequently been reported in schizophrenia as well as in attention deficit hyperactivity disorder (ADHD). This study represents an attempt to compare groups of adolescents with schizophrenia and ADHD on a comprehensive neuropsychological test battery. Such a comparison affords the opportunity to ascertain differences in the degree, profile, and specificity of impairments. METHOD: The performance of 19 adolescents with schizophrenia, 20 adolescents with ADHD, and 30 normal adolescents on a broad battery of cognitive tests was compared. RESULTS: The schizophrenic group showed the most pronounced deficits on tests of abstraction, visual memory, and motor function in comparison with the subjects with ADHD, while the ADHD subjects had the most pronounced deficits on measures of attention, verbal memory, and learning. CONCLUSIONS: The subjects with schizophrenia appeared to have a more general pattern of brain dysfunction, whereas the impairment of the ADHD subjects seemed to be relatively specific to tests associated with frontal lobe function.
In schizophrenia and attention deficit hyperactivity disorder (ADHD), the major symptoms represent primarily cognitive dysfunction (1–8). However, these two disorders have attentional dysfunction as the only common symptom. On this basis, one may assume that at least one common area of the brain is dysfunctional in both patient groups, while several other dysfunctional brain areas are not common for the two disorders. By studying which neuropsychological functional domains are affected in these disorders, one may also attempt to ascertain which areas in the brain are affected. This may eventually provide hypotheses about which areas and connections in the brain are dysfunctional in relation to the shared symptoms of these two disorders (some attentional deficits) and which brain dysfunctions are fundamental for the development of different symptoms (psychotic symptoms and hyperactivity).
To our knowledge, no previous study has directly compared the cognitive performance of adolescents with schizophrenia and adolescents with ADHD. Some investigators have noted similarities in cognitive function in offspring of subjects with schizophrenia and children with ADHD (9, 10). Marcus and associates (10) found that the motor, perceptual, and attentional difficulties observed in offspring of subjects with schizophrenia resembled the syndrome of behavioral patterns defined as attention deficit disorder in DSM-III. In previous articles from our research group, we reported increased vulnerability to masking stimuli when a backward-masking task was used in a group of adolescents with schizophrenia and in a group of adolescents with ADHD (11); however, no sustained attention deficits were observed in either of the groups (12). Other investigators have demonstrated distinctly different deficits in sustained attention between children of subjects with schizophrenia and children with attention deficit disorder (1, 2). Asarnow et al. (13) concluded that the amount of information to be processed in a partial-report span of apprehension task appeared to have a greater effect on the performance of children with schizophrenia than on that of children with attention deficit disorder.
The few studies that have compared subjects with schizophrenia and subjects with ADHD have examined children and have concentrated on one or few areas of cognition, rather than using a comprehensive test battery that would permit adequate profiling of test results across neuropsychological dimensions. This study represents the first attempt, we believe, to compare these two groups of subjects on a comprehensive neuropsychological test battery.
METHOD
Subjects
A total of 69 adolescents participated in this study: 19 with a DSM-III-R diagnosis of a schizophrenic disorder, 20 with a DSM-III-R diagnosis of ADHD, and 30 healthy volunteer comparison subjects. The schizophrenic group consisted of five female and 14 male patients whose mean age was 16.2 years (SD=1.1). The ADHD group consisted of 20 male patients whose mean age was 14.1 years (SD=1.5), and the normal group consisted of 14 female and 16 male subjects whose mean age was 15.7 years (SD=1.6). The ADHD group was significantly younger than the two other groups (F=11.52, df=2, 66, p<0.001). Eleven of the subjects with schizophrenia and all of the ADHD subjects were recruited from the National Centre for Child and Adolescent Psychiatry in Oslo. Eight subjects with schizophrenia were recruited from other hospitals in the region. Fifteen of the subjects with schizophrenia were inpatients. They had been hospitalized approximately 12 weeks (mean=11.7, SD=15.6). Four outpatients with schizophrenia had never been inpatients. All of the ADHD subjects were outpatients. The subjects in the normal group attended regular school classes at normal grade level; these subjects received a small compensation to cover their traveling costs. The Child Behavior Checklist (14) was used to screen for mental problems, and individuals with a total raw score higher than 45 were excluded. The checklist is a general questionnaire to be filled out by parents, generating eight syndrome scale scores.
The diagnosis of schizophrenia and the subtype were assigned on the basis of clinical interviews by senior clinicians (including B.R.R.) and the patients’ case records. Consensus research diagnoses were made for a subgroup of 13 patients, and two senior psychologists (one of whom was B.R.R.) agreed on the schizophrenia diagnosis in 12 (92%) of the cases. Schizophrenic patients received the following subtype diagnoses: disorganized (N=11), paranoid (N=4), undifferentiated (N=1), schizophreniform disorder (N=1), schizoaffective disorder (N=1), and delusional disorder (N=1). The patients were reassessed 1 year later, and the diagnosis was confirmed in each case.
Diagnostic evaluations for the ADHD group were made according to semistructured clinical interviews and standardized rating scales. The adolescents met at least eight of the DSM-III-R criteria for the condition. There were marked attention problems both at home and at school. In addition, all of these subjects had shown substantial hyperactivity, impulsivity, and inattention between ages 6 and 10 as assessed by the Parent’s Rating Scale (15). None of these patients had a history of psychosis. Patients received the following comorbidity diagnoses: oppositional defiant disorder (N=9), developmental reading disorder (N=2), both oppositional defiant disorder and developmental reading disorder (N=3).
Eleven patients in the schizophrenic group and eight patients in the ADHD group were drug-naive. Five of the patients with schizophrenia were receiving standard neuroleptic medication (perphenazine, N=3; thioridazine, N=1; zuclopenthixol, N=1) at the time of testing. Three of the patients with schizophrenia were drug-free during testing and for a period of at least 5 days before testing. The mean defined daily dose of neuroleptic medication was 0.7 (SD=0.3) (defined daily dose is the assumed average maintenance dose per day for a drug used for its main indication in adults [Drug Consumption in Norway, Norwegian Medical Depot, 1993]). The neuroleptic-naive patients had been hospitalized for a mean of 6.4 weeks (SD=6.2), while the 12 patients who used medication or had used medication 5 days before testing had been hospitalized for a mean of 17.5 weeks (SD=21.8). Twelve of the subjects with ADHD received stimulant medication (methylphenidate, N=11; dextroamphetamine, N=1). The medication was discontinued for at least 24 hours before testing. One patient with ADHD received a small dose of haloperidol (1 mg/day) because of tics.
Scores on the Brief Psychiatric Rating Scale (BPRS) (16) were obtained for 11 of the patients with schizophrenia within 1 week of testing. Interrater reliability (intraclass correlation coefficient 1, 2 [17]) for the BPRS total score was 0.99. A positive symptom score and a negative symptom score based on a factor analysis conducted by Ventura et al. (unpublished paper, 1995) were extracted from the BPRS scores. The scores showed that the patients were characterized by psychotic symptoms at the time of testing and by more positive than negative symptoms. The following numbers of these 11 patients had a score of more than 3 on variables in the positive symptom score: unusual thought content, N=9; grandiosity, N=3; hallucinations, N=7; conceptual disorganization, N=3; bizarre behavior, N=4; suspiciousness, N=7; disorientation, N=2. The mean total score on the BPRS in the schizophrenic group was 61.1 (SD=19.3, range=31–90), the mean positive symptom score was 21.2 (SD=7.5, range=9–30), and the mean negative symptom score was 4.6 (SD=3.4, range=3–9). The mean score on the Global Assessment Scale (GAS) (18) was 41.7 (SD=12.0) in the schizophrenic group and 50.8 (SD=7.5) in the ADHD group. The patients with schizophrenia scored significantly lower on the GAS than the patients with ADHD (F=8.10, df=1, 36, p=0.007). The mean raw score on the Child Behavior Checklist was 61.8 (SD=32.8) in the schizophrenic group, 60.3 (SD=17.7) in the ADHD group, and 13.4 (SD=10.2) in the normal comparison group. The scores on the Child Behavior Checklist showed that the level of psychopathology in the patient groups was significantly above that in the normal comparison group (F=45.80, df=2, 62, p<0.001). (For more detailed information on the BPRS, GAS, and Child Behavior Checklist, see Rund et al. [12].)
All subjects were screened for a history of head injury with loss of consciousness, substance abuse, a general medical condition that was likely to affect CNS functions, and mental retardation. The estimated average IQ, as determined by the similarities, block design, digit span, and digit symbol subtests of the Wechsler Intelligence Scale for Children—Revised (WISC-R) (19), was 98.22 (SD=11.38) in the schizophrenic group, 98.62 (SD=11.24) in the ADHD group, and 110.54 (SD=9.49) in the normal group; the patient groups scored significantly lower than the normal group (F=11.17, df=2, 66, p<0.001). One subject in the schizophrenic group, three subjects in the ADHD group, and one subject in the normal group were left-handed.
Neuropsychological Test Battery
All subjects were tested with a comprehensive neuropsychological test battery designed to assess the following functional domains: abstraction-flexibility, spatial organization, visual memory, verbal memory and learning, speeded visual-motor processing and attention, motor function, auditory processing-distractibility, sustained attention, apprehension, and selective attention. The test battery included (in order of administration) the Wisconsin Card Sorting Test (20), the digit span subscale of the WISC-R (19), dichotic listening (21, 22), the digit symbol subscale of the WISC-R, digit symbol location (23), the California Verbal Learning Test, trials 1–5 (24), Kimura recurring figures (25), the Seashore rhythm test (26), the digit span distractibility test (27), the California Verbal Learning Test, long delay and recognition (24), the Trail Making Test, parts A and B (26), a partial-report span of apprehension test (Asarnow and Nuechterlein, unpublished), the similarities subscale of the WISC-R, the degraded stimulus Continuous Performance Test (1, 12), the block design subscale of the WISC-R, the Sustained Attention Test (28), and the grooved pegboard (26).
After a complete description of the study to the subjects, written informed consent was obtained. The consent form was signed by both the adolescent and a parent. The subjects were tested individually. All subjects received the same battery of tests in a fixed order. Total time for the test battery was about 4 hours, including breaks.
Data Analysis
Raw test scores (Table 1) were transformed to standard equivalents (z scores) on the basis of the means and standard deviations of the normal comparison group. Test data were reduced to 10 summary measures by averaging each subject’s z scores on tests assessing the same functional domain. The organization of tests indicated in Table 2 is modified according to Saykin et al. (4, 5).
Where high scores indicated impairment, scores were transformed (direction reversed) so that high scores always indicated better cognitive function. The 10 summary measures were used as dependent variables in a multivariate analysis of covariance (MANCOVA) with diagnostic group (schizophrenic, ADHD, and normal) serving as the between-group factor, covarying for age because the ADHD group was significantly younger than the two other groups. This was followed by univariate analyses of covariance (ANCOVAs) with age as the covariate for each summary measure. Significant univariate effects were followed by post hoc comparisons (Scheffé test) between each pair of groups.
When the Levene test for homogeneity of variance (29) revealed significant differences between groups, nonparametric tests (Kruskal-Wallis, Spearman rank correlation) were performed on the variables, in addition to standard ANCOVAs. Results are commented on if they altered the findings.
A within-subject contrast on each summary measure was conducted. For each contrast, the score for a particular summary measure was compared with the mean of the remaining summary measures in the profile. This procedure permitted determination of areas of selective deficit, as opposed to generalized impairment equally affecting all functions, by testing for between-group differences in profile shape (4). For significance testing of the contrasts, we used pairwise t tests.
Pearson’s product-moment correlations and Spearman’s rank correlations were used to test associations between the summary scores and age and between the summary scores and sex.
To assess the number of adolescent patients with clinically significant impairment, a threshold of 2 SD from the normal subjects’ mean was used.
RESULTS
The z score profile on the neuropsychological test battery is presented in Figure 1. By definition, the normal comparison group mean is represented by the zero line, with SD=1 for all functions. As Figure 1 shows, there was a clear difference in performance pattern across groups, with the overall MANCOVA being significant (Wilks’s lambda F=3.13, df=20, 104, p<0.0001). ANCOVAs on the summary scores revealed a significant difference between groups on abstraction-flexibility (F=7.05, df=2, 62, p=0.002), auditory processing-distractibility (F=7.08, df=2, 65, p=0.002), apprehension (F=4.20, df=2, 65, p=0.02), motor processing (F=8.62, df=2, 65, p<0.001), spatial organization (F=3.68, df=2, 65, p=0.03), visual memory (F=16.45, df=2, 64, p<0.001), visual-motor processing and attention (F=11.34, df=2, 65, p<0.001), and verbal memory and learning (F=8.65, df=2, 65, p<0.001). There were no significant differences between groups on selective attention (F=0.40, df=2, 65) or sustained attention (F=1.30, df=2, 65). Post hoc comparisons indicated that the schizophrenic group had significantly lower scores than the normal subjects on abstraction-flexibility, visual-motor processing and attention, visual memory, verbal memory and learning, spatial organization, and motor function. The ADHD subjects scored significantly lower than the normal subjects on verbal memory and learning, visual-motor processing and attention, auditory processing-distractibility, and apprehension. The schizophrenic group scored significantly lower than the ADHD group on visual memory.
The Levene test for homogeneity of variance revealed significant differences between groups on most of the summary scores. However, the results did not change significantly when the analysis was done with nonparametric tests.
Age was positively correlated with sustained attention (rs=0.31, N=69, p=0.01).
The planned profile contrasts indicated that motor function and visual memory were selective areas of deficit for the patients with schizophrenia (t=2.28, df=17, p=0.04, and t=3.18, df=17, p=0.005, respectively), whereas auditory processing-distractibility were selective areas of deficit for the patients with ADHD (t=3.24, df=19, p=0.004). The functional domains that were the least impaired in the schizophrenic group were sustained attention (t=–5.9, df=17, p<0.001) and selective attention (t=–3.44, df=17, p=0.003), and the least impaired functional domain in the ADHD group was selective attention (t=–3.25, df=19, p=0.004). The remaining functional domains were not significantly different from the profile mean.
The percentage of patients with schizophrenia who performed more than 2 SD below the mean of the normal subjects was greater for visual memory (42%) and abstraction-flexibility (32%) than for the measures of other functions (range=5%–26%). In the ADHD group, 30% performed more than 2 SD below the mean of the normal subjects on auditory processing-distractibility and speeded visual-motor processing and attention. The proportion of ADHD subjects performing more than 2 SD below the mean of the normal subjects on other functions ranged from 5% to 25%.
Univariate ANCOVAs, with age as the covariate, on components from a principal-component analysis and post hoc t tests (p<0.05, Scheffé test) revealed that the schizophrenic group scored significantly lower than the ADHD group and the normal group on a component score comprising visual memory, abstraction-flexibility, speeded visual-motor processing and attention, and motor function (F=20.79, df=2, 61, p<0.0001). The ADHD group scored significantly lower than the schizophrenic and normal groups on a component score comprising sustained attention, apprehension, auditory processing-distractibility, and verbal memory and learning (F=4.98, df=2, 61, p<0.01).
The analyses were recomputed without the data from the three patients with a diagnosis outside the conservative definition of schizophrenia. The analyses were also recomputed without the data from the subjects with developmental reading disorder in the ADHD group. None of these recomputations changed the results significantly. Further, the results did not change significantly when the analyses were recomputed excluding data from the five patients with schizophrenia who were using neuroleptic medication.
DISCUSSION
Both the adolescents with schizophrenia and those with ADHD showed significant neuropsychological impairment in comparison with healthy adolescents. However, the pattern of deficits was quite different for the two patient groups. The performance of the schizophrenic subjects was significantly more deficient than that of the subjects with ADHD on a test of visual memory. Further, the patients with schizophrenia had significantly lower scores than the normal subjects on abstraction-flexibility, visual memory, spatial organization, and motor function. In contrast, the subjects with ADHD scored significantly worse than the normal subjects on auditory processing-distractibility and apprehension. Planned contrast analysis, percent analysis, and ANCOVAs on components from a principal-component analysis supported the pattern of different impaired cognitive functions in patients with schizophrenia compared with patients with ADHD.
The schizophrenic group showed selective impairment in visual memory in comparison with the ADHD group, suggesting that the visual memory deficit is specifically related to schizophrenia. Our results suggest that adolescents with schizophrenia show evidence of mnestic disturbances that are not related to attentional dysfunctions (see reference 30 for further discussion).
Subjects in the schizophrenic group performed more poorly than the ADHD subjects on the Wisconsin Card Sorting Test, which requires abstraction-flexibility. It has been proposed that impairment of complex problem-solving ability reflects dysfunction in the “executive” control of working memory. Working memory is defined as a multicomponent system that allows for the simultaneous storage and processing of information (31). The Wisconsin Card Sorting Test is assumed to be an executive task that involves planning and problem solving and requires new concept learning for optimal performance (21). The specific attentional tasks on which ADHD subjects performed poorly (digit span distractibility test) also involve working memory. However, the Wisconsin Card Sorting Test requires more shifting of set and thus a greater contribution from working memory. The deficient performance of the schizophrenic group in comparison with the ADHD group on tests of abstraction-flexibility may reflect a larger failure in working memory, while the lower performance of the ADHD subjects in comparison with the schizophrenic subjects on tests of auditory processing-distractibility may indicate that they were having more difficulties in scanning and selecting relevant aspects of complex stimuli situations.
The greater distractibility in the ADHD group and the impairment in abstraction-flexibility in the schizophrenic group suggest dysfunction within the frontal circuit in both groups. This is consistent with a large body of neuropsychological data (8) and a growing body of brain imaging data (32, 33) suggesting that frontal lobe dysfunction is characteristic of ADHD and with magnetic resonance imaging studies demonstrating frontal lobe abnormalities in schizophrenia (34, 35).
Consistent with previous neuropsychological studies of schizophrenia, our schizophrenic group demonstrated impairments in comparison with normal subjects on measures of abstraction, mental flexibility, visual memory, verbal memory, learning (4, 5), and psychomotor function (3, 36, 37). These deficits have also been reported in first-episode schizophreniform patients (38). The present results show that these impairments are also apparent in adolescents with schizophrenia, the majority of whom were neuroleptic-naive. The weak performance of the subjects with ADHD in comparison with the normal subjects on various tasks of attention, verbal memory, and learning confirm earlier reports on neuropsychological function in adolescents with ADHD (6–8).
Neither adolescents with schizophrenia nor adolescents with ADHD showed any dysfunction on tests of visual sustained attention and auditory selective attention or auditory laterality. This is in contrast to the findings of some previous studies (6, 39, 40). The ADHD subjects in the present study were older than children with ADHD examined in any previous studies on vigilance deficits. Decline in level of hyperactivity and improvement in attention span and impulse control have been documented in adolescents with ADHD (41). It is possible that the adolescents with ADHD in the present study showed deficits in vigilance (degraded stimulus Continuous Performance Test) in an earlier phase of the disorder but that these deficits declined with age. Further, our findings on this test may be seen as a confirmation of Corkum and Siegel’s (42)conclusion that there is no compelling evidence for sustained attention deficit in ADHD subjects. The performance of the schizophrenic group was not significantly deficient in comparison with that of the normal group on measures of apprehension or auditory processing-distractibility (12, 30).
In contrast to the ADHD subjects, the adolescents with schizophrenia were younger than most of the subjects in previous studies of attention deficits in schizophrenia. They were in an early phase of the illness, when psychosis had not yet become established, and may show impairments in vigilance and/or selective attention later. Further, most of them were neuroleptic-naive and had been hospitalized for only a short period. The present results may indicate that deficits in these attentional domains, observed in other patients with schizophrenia, reflect effects of the illness process, neuroleptic medication, and/or hospitalization. Asarnow and colleagues (13) found that children with schizophrenia performed poorly on tasks that require sustained attention and apprehension. Kenny et al. (43) found deficits on measures of attention in their group of adolescents with schizophrenia. However, the patients in these two studies did not perform the same attention task as in our study, and they were stabilized on neuroleptic medication regimens at testing.
In the present study, the subjects with ADHD differed from the subjects with schizophrenia on several dimensions, which could confound interpretation of the findings. As opposed to the subjects in the schizophrenic group, the ADHD subjects were all male, were significantly younger, and had higher GAS scores, and none were inpatients. These factors tend to make comparisons between the two groups more questionable. However, ADHD subjects are typically young males who have never been hospitalized, with better psychosocial functioning than subjects with schizophrenia. We would therefore claim that our group of ADHD patients is representative of this patient group and that the within-group findings are reliable. Age served as a covariate in the analyses. However, this may not have entirely controlled for the effect of age differences on the results.
One limitation of the present study is that the majority of the subjects with schizophrenia and all of the subjects with ADHD were male. Thus, it is uncertain whether the findings would be the same if more female subjects were to be included.
Comorbidity is a problem within the group of ADHD subjects. A substantial number of the ADHD subjects in our group met the DSM-III-R criteria for oppositional defiant disorder and for developmental reading disorder in addition to the ADHD diagnosis. However, it is common to find elevated rates of oppositional defiant disorder (43%–93%) (44) and developmental reading disorder (20%–40%) (45) in groups of subjects with ADHD. Impairment in verbal memory and learning could be related to the subjects’ reading disorder and not to ADHD. However, when we excluded the data on the subjects with reading disorders from the analysis, the results did not change significantly.
Another factor that could have affected the neuropsychological test scores of the schizophrenic group was neuroleptic medication. However, only five subjects received such medication. Furthermore, follow-up analyses revealed no significant differences in any area of neuropsychological ability between patients receiving and not receiving medication.
Neuropsychological tests are only indirect measures of brain function. Further exploration of relationships between neuropsychological status and other evidence of structural and functional brain abnormalities is needed.
In the profile contrasts, there were 10 comparisons. This may have resulted in an inflated type I error rate because the 10 variables are correlated with each other. However, there is no generally accepted way to resolve this problem. More conservative criteria for significance might have increased the possibility of making a type II error in this study with few subjects.
The selectivity of observed deficits was not analyzed using the standardized residual score recommended by Chapman and Chapman (46). Most of the tasks were not matched for difficulty level, and some of the tasks may not have been complex enough to differentiate between the groups. However, a strength of the study is that the design permitted the analysis of specific deficits within the broader context of a performance profile of different cognitive and motor composites, rather than viewing deficits in isolation, which carries the risk of drawing inaccurate conclusions about deficit selectivity.
In summary, the results in this study suggest that patients with schizophrenia have poorer mental flexibility than patients with ADHD, whereas inability to control attention, or distractibility, appears to be more central to the neuropsychology of ADHD. These differences in neuropsychological profile support a hypothesis that the cognitive impairments in schizophrenia and ADHD are due to disruptions of different neurobiological substrates. Subjects with schizophrenia appear to have a more general pattern of brain dysfunction, whereas the impairment of ADHD subjects seems to be relatively specific to tests associated with frontal lobe function. Further research on larger study groups must compare these two types of patients on cognitive tests to clarify whether the differences in cognitive function found in the present study are fundamental for the development of the different symptoms (psychotic symptoms and hyperactivity), and whether reduced activity within the frontal circuit, as implied by working memory deficits in the two patient groups, reflects the observed behavioral attention problems that these two groups seem to have in common.
Received July 31, 1998; revisions received Dec. 7, 1998, and Jan. 21, 1999; accepted Feb. 9, 1999. From the National Centre for Child and Adolescent Psychiatry, University of Oslo; and the Institute of Psychology, University of Oslo. Address reprint requests to Dr. Rund, Institute of Psychology, University of Oslo, P.O. Box 1094, Blindern, N-0317 Oslo, Norway Supported by grants from the Norwegian Research Council (number 377.94/013), Haldis and Josef Andresen’s Foundation, Anders Jahre’s Fund for the Promotion of Science, Eilertsen’s Legacy, and the Nansen Fund. The authors thank Terry E. Goldberg, Ph.D., for comments on the manuscript.
 |
 |
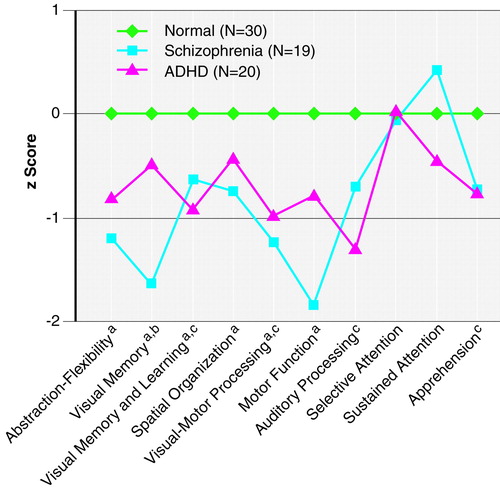
FIGURE 1. Attention, Memory, and Executive Function of Adolescents With Schizophrenia, Adolescents With Attention Deficit Hyperactivity Disorder (ADHD), and Normal Comparison Adolescents
The schizophrenic group performed significantly worse than the normal group (p<0.05, post hoc Scheffé test).
The schizophrenic group performed significantly worse than the ADHD group (p<0.05, post hoc Scheffé test).
The ADHD group performed significantly worse than the normal group (p<0.05, post hoc Scheffé test).
1. Nuechterlein KH: Signal detection in vigilance tasks and behavioral attributes among offspring of schizophrenic mothers and among hyperactive children. J Abnorm Psychol 1983; 92:4–28Crossref, Medline, Google Scholar
2. Nuechterlein KH, Dawson ME: Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophr Bull 1984; 10:160–203Crossref, Medline, Google Scholar
3. Goldberg TE, Weinberger DR, Berman KF, Pliskin NH, Podd MH: Further evidence for dementia of the prefrontal type in schizophrenia? Arch Gen Psychiatry 1987; 44:1008–1014Google Scholar
4. Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, Kester DB, Stafiniak P: Neuropsychological function in schizophrenia: selective impairment in memory and learning. Arch Gen Psychiatry 1991; 48:618–624Crossref, Medline, Google Scholar
5. Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC: Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry 1994; 51:124–131Crossref, Medline, Google Scholar
6. Douglas VI: Stop, look and listen! The problem of sustained attention and impulse control in hyperactive and normal children. Can J Behav Sci 1972; 4: 259–282Google Scholar
7. Douglas VI: Attention and cognitive problems, in Developmental Neuropsychiatry. Edited by Rutter M. New York, Guilford Press, 1983, pp 280–329Google Scholar
8. Barkley RA, Grodzinsky G, DuPaul GJ: Frontal lobe functions in attention deficit disorder with and without hyperactivity: a review and research report. J Abnorm Child Psychol 1992; 20:163–188Crossref, Medline, Google Scholar
9. Bellak L: Psychiatric aspects of minimal brain dysfunction in adults: their ego function assessment, in Psychiatric Aspects of Minimal Brain Dysfunction in Adults. Edited by Bellak L. New York, Grune & Stratton, 1979, pp 73–102Google Scholar
10. Marcus J, Hans SL, Nagler S, Auerbach JG, Mirsky AF, Aubrey A: Review of the NJMH Israeli kibbutz-city study and the Jerusalem infant development study. Schizophr Bull 1987; 13:425–438Crossref, Medline, Google Scholar
11. Rund BR, Øie M, Sundet K: Backward-masking deficit in adolescents with schizophrenic disorders or attention deficit hyperactivity disorder. Am J Psychiatry 1996; 153:1154–1157Google Scholar
12. Rund BR, Zeiner P, Sundet K, Øie M, Bryhn G: No vigilance deficit found in either young schizophrenic or ADHD subjects. Scand J Psychol 1998; 39:101–107Crossref, Medline, Google Scholar
13. Asarnow RF, Asamen J, Granholm E, Sherman T, Watkins JM, Williams ME: Cognitive/neuropsychological studies of children with a schizophrenic disorder. Schizophr Bull 1994; 20:647–669Crossref, Medline, Google Scholar
14. Achenbach TM: Manual for the Child Behavior Checklist/4-18 and 1991 Profile. Burlington, University of Vermont, Department of Psychiatry, 1991Google Scholar
15. Wender PH, Wood DR, Reimherr FW: Pharmacological treatment of attention deficit disorder, residual type (ADD, RT, “minimal brain dysfunction,” “hyperactivity”) in adults. Psychopharmacol Bull 1985; 21:222–231Medline, Google Scholar
16. Lukoff D, Nuechterlein KH, Ventura J: Appendix A: Manual for Expanded Brief Psychiatric Rating Scale (BPRS). Schizophr Bull 1986; 12:594–602Google Scholar
17. Schrout PE, Fleiss JC: Intraclass correlations, used in assessing rater reliability. Psychophysiology 1979; 8:191–198Google Scholar
18. Endicott J, Spitzer RL, Fleiss J, Cohen J: The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 1976; 33:766–771Crossref, Medline, Google Scholar
19. Wechsler D: WISC-R Manual: Wechsler Intelligence Scale for Children—Revised. New York, Psychological Corp, 1974Google Scholar
20. Heaton RK: Wisconsin Card Sorting Test, Manual. Odessa, Fla, Psychological Assessment Resources, 1981Google Scholar
21. Hugdahl K, Andersson L: The “forced-attention” paradigm in dichotic listening to CV-syllables: a comparison between adults and children. Cortex 1986; 22:417–432Crossref, Medline, Google Scholar
22. Øie M, Rund BR, Sundet K, Bryhn G: Auditory laterality and selective attention: normal performance in patients with early-onset schizophrenia. Schizophr Bull 1998; 24:643–652Crossref, Medline, Google Scholar
23. Kaplan E, Fein D, Morris R, Delis DC: WAIS-R as a neuropsychological instrument. San Antonio, Tex, Psychological Corp, 1991Google Scholar
24. Delis DC, Kramer JH, Kaplan E, Ober BA: The California Verbal Learning Test (CVLT) Manual. New York, Psychological Corp, 1987Google Scholar
25. Kimura D: Right temporal lobe damage. Arch Neurol 1963; 8:264–271Crossref, Medline, Google Scholar
26. Lezak MD: Neuropsychological Assessment. New York, Oxford University Press, 1995Google Scholar
27. Oltmanns TF, Neale JM: Schizophrenic performance when distractors are present: attention deficit or differential task difficulty? J Abnorm Psychol 1975; 84:205–209Google Scholar
28. Rund BR, Ørbeck AL, Landrø NI: Vigilance deficits in schizophrenics and affectively disturbed patients. Acta Psychiatr Scand 1992; 86:207–212Crossref, Medline, Google Scholar
29. Norusis MJ: SPSS for Windows: Base System User’s Guide, Release 6.0. Chicago, SPSS, 1993Google Scholar
30. Øie M, Sundet K, Rund BR: Contrast in memory function between adolescents with schizophrenia or ADHD. Neuropsychologia (in press)Google Scholar
31. Baddeley AD: Working Memory. New York, Oxford University Press, 1986Google Scholar
32. Hynd GW, Semrud-Clinkeman M, Lorys AR, Novey ES, Eliopulos D: Brain morphology in developmental dyslexia and attention deficit/hyperactivity. Arch Neurol 1990; 47:919–926Crossref, Medline, Google Scholar
33. Semrud-Clinkeman M, Filipek PA, Biederman J, Steingard R, Kenny D, Renshaw P, Bekken K: Attention deficit hyperactivity disorder: magnetic resonance imaging morphometric analysis of the corpus callosum. J Am Acad Child Adolesc Psychiatry 1994; 33:875–881Crossref, Medline, Google Scholar
34. Andreasen NC, Erhardt JC, Swayze VW II, Alliger RJ, Yuh WT, Cohen G, Ziebell S: Magnetic resonance imaging of the brain in schizophrenia: the pathophysiologic significance of structural abnormalities. Arch Gen Psychiatry 1990; 47:35–44Crossref, Medline, Google Scholar
35. Seidman LJ, Yurgelin-Todd D, Kremen WS, Woods BT, Goldstein JM, Faraone SV, Tsuang MT: Relationship of prefrontal and temporal lobe MRI measures to neuropsychological performance in chronic schizophrenia. Biol Psychiatry 1994; 35:235–246Crossref, Medline, Google Scholar
36. Goldstein G: The neuropsychology of schizophrenia, in Neuropsychological Assessment of Neuropsychiatric Disorders. Edited by Grant I, Adams K. New York, Plenum, 1986, pp 147–171Google Scholar
37. Sullivan EV, Shear PK, Zipurky RB, Harvey JS, Pfefferbaum A: A deficit profile of executive, memory and motor functions in schizophrenia. Biol Psychiatry 1994; 36:641–653Crossref, Medline, Google Scholar
38. Hoff AL, Riordan H, O’Donnell DW, Morris L, DeLisi LE: Neuropsychological functioning of first-episode schizophreniform patients. Am J Psychiatry 1992; 149:898–903Link, Google Scholar
39. Green MF, Hugdahl K, Mitchell S: Dichotic listening during auditory hallucinations in patients with schizophrenia. Am J Psychiatry 1994; 151:357–362Link, Google Scholar
40. Nuechterlein KH, Buchsbaum MS, Dawson ME: Neuropsychological vulnerability to schizophrenia, in The Neuropsychology of Schizophrenia. Edited by David AS, Cutting JC. Hillsdale, NJ, Lawrence Erlbaum Associates, 1994, pp 53–77Google Scholar
41. Barkley RA: Attention Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. New York, Guilford Press, 1990Google Scholar
42. Corkum PV, Siegel LS: Is the Continuous Performance Task a valuable research tool for use with children with attention-deficit-hyperactivity disorder? J Child Psychol Psychiatry 1993; 34:1217–1239Google Scholar
43. Kenny JT, Friedman L, Findling RL, Swales TP, Strauss ME, Jesberger JA, Schultz SC: Cognitive impairment in adolescents with schizophrenia. Am J Psychiatry 1997; 154:1613–1615Google Scholar
44. Jensen PS, Martin D, Cantwell DP: Comorbidity in ADHD: implications for research, practice, and DSM-V. J Am Acad Child Adolesc Psychiatry 1997; 36:1065–1079Google Scholar
45. Barkley RA, DuPaul G, McMurray MB: A comprehensive evaluation of attention deficit disorder with and without hyperactivity as defined by research criteria. J Consult Clin Psychol 1990; 58:775–789Crossref, Medline, Google Scholar
46. Chapman LJ, Chapman JP: Strategies for resolving the heterogeneity of schizophrenics and their relatives using cognitive measures. J Abnorm Psychol 1989; 98:357–366Crossref, Medline, Google Scholar



