Accumulation of Macrophages in the CSF of Schizophrenic Patients During Acute Psychotic Episodes
Abstract
OBJECTIVE: There have been numerous reports of organic or structural abnormalities in the central nervous system (CNS) of patients with schizophrenia. Given that pathological conditions in the CNS are frequently reflected in the cell profiles of CSF, the authors compared the cytology of CSF from schizophrenic patients with that from a reference population in order to find out trails of elementary pathogenetic events in this serious psychiatric disease. METHOD: CSF samples from 35 patients with acute schizophrenia and 46 comparison subjects were prepared by Millipore filtration. The total and differential counts of CSF mononuclear cells were performed by light microscopy. RESULTS: At the beginning of treatment, the proportion of mononuclear phagocytes/macrophages in the patients’ CSF was significantly higher than that in the comparison subjects. During treatment with conventional neuroleptic medication, the cytology returned to normal in several patients. CONCLUSIONS: The high proportion of macrophages in schizophrenia without a significantly higher total cell count may reflect neurodevelopmental disorder, a neurodegenerative process, or subtle CNS immunoactivation with mobilization of microglia.
The etiopathogenesis of schizophrenia has remained elusive. Several findings have, however, suggested that organic factors contribute to the pathogenesis of the disease. Among these, hereditary factors (1), neurodevelopmental disturbances (2, 3), and immunological aberrations (4–7) have been proposed to be of significance. The attractiveness of the dopamine theory has been refreshed by reports on abnormal expression of the D4 and D5 receptors (8, 9). Abnormalities in the sites of serotonin (5-HT) uptake (10) and 5-HT receptors (11) have also been implicated. The action of atypical antipsychotic drugs such as clozapine and risperidone have been supposed to be related to their 5-HT2 antagonism (12). Modern neuroimaging techniques have disclosed low volume (13–15) and gray matter deficits (16, 17), especially concerning limbic structures in the medial temporal lobe surrounding the temporal horn (18, 19), in the brains of schizophrenic patients. Skewings of circulating and CSF cytokine levels and production have been detected; the affected cytokines include interleukin 2 (IL-2) (20, 21), IL-6 (22), and tumor necrosis factor alpha (23).
These findings have raised the question of whether the abnormalities originate from developmental, degenerative, inflammatory, or immunoactive processes in the central nervous system (CNS).
Experimental models of nerve injury have revealed the critical role of macrophages in both degenerative and regenerative actions in the nervous system (24, 25). In certain neurological diseases, where patients suffer from neuronophagia, the activity of resident macrophages (from microglial or perivascular origin) seems to contribute to the disappearance of neurons (26).
In this study we investigated whether reflections of cytological abnormalities in the CNS are detectable in the CSF of patients with acute attacks of schizophrenia, knowing that the CSF cell distribution of normal adults is one-third mononuclear phagocytes/macrophages and two-thirds lymphocytes and that deviations from normal cell counts or relative cell compositions frequently occur in CNS diseases (27–29).
METHOD
Subjects
Thirty-five patients, 19 male and 16 female, admitted to Hesperia Hospital (Helsinki City Hospital) in the acute phase of schizophrenia took part in the study. After complete description of the study to the subjects, written informed consent was obtained. The mean age of the male patients was 34 years (SD=9) and that of the female patients was 32 years (SD=11). For 20 patients it was the first admission, and they had not taken neuroleptic medication previously. The remaining 15 patients had been treated previously for at least one psychotic episode but had been drug free for more than 4 months before the current admission. The DSM-III-R diagnostic criteria were applied to confirm that the psychosis was of the schizophrenic type (schizophrenia or schizophreniform psychosis). Acute infections were excluded clinically and by routine laboratory infection markers in peripheral blood and CSF. The CSF-serum ratio for albumin was within reference limits in all subjects, thus excluding significant damage to the blood-brain barrier.
The reference population consisted of subjects previously examined for nonspecific neurological symptoms, such as headache and vertigo, at the Helsinki University Hospital, Department of Neurology. A total of 46 individuals, 21 male (mean age=33 years, SD=8) and 25 female (mean age=32 years, SD=9), were accepted as comparison subjects after a 2-year follow-up period with no evidence of inflammatory or CNS disease (30).
Cytological Techniques
CSF samples were collected within a few days (mean=4, range=0–7) of hospital admission. Thirteen patients each gave a second sample after a few weeks of neuroleptic treatment. One milliliter of CSF was fixed in 1 ml of 96% ethanol, and the specimens were prepared by Millipore filtration (according to 1976 catalogue and purchasing guide from Millipore, Bedford, Mass.). In addition to the Millipore filtration method, cytocentrifuged (800 rpm for 8–10 minutes) CSF samples from patients were stained by the May-Gr�-Giemsa method and analyzed by light microscopy in order to observe detailed morphological features of the cells and to exclude samples with red blood cell (RBC) contamination; samples with more than 20 RBCs per high power (40×) field were rejected. The methodological details have been previously described (29, 31, 32).
Statistical Methods
The Mann-Whitney U test was used to study differences in the total cell count and lymphocyte/macrophage distributions between the patient and comparison groups. The Wilcoxon matched pairs test was the statistical method applied to compare the patients’ CSF cytology before and after treatment. F tests were performed by using rank transforms and analysis of variance (ANOVA).
RESULTS
Cytological examination of the CSF from the 35 psychotic patients revealed that they had a significantly higher proportion of cells morphologically classified as mononuclear phagocytes/macrophages than was present in the CSF of the comparison subjects (F=47.34, df=1, 79, p<0.0001). The total CSF cell count was not, however, significantly higher in the patient group.
The cytological details of the phagocytes frequently included a kidney-shaped or lobulated nucleus and a voluminous cytoplasm with numerous small vacuoles. Overt lipophages with larger cytoplasmic vacuoles, typically seen in the CSF after acute brain injuries, for instance, were rarely seen (Figure 1).
A tendency toward normalization of the cytological picture (i.e., decrease in the proportion of macrophages) was observed in the CSF of the 13 psychotic patients who took part in the follow-up section of the study (Wilcoxon matched pairs test, rank transforms, and repeated measures ANOVA: F=7.83, df=1, 12, p<0.05) after a few weeks of treatment with typical neuroleptics (Figure 2).
DISCUSSION
There is a growing body of evidence that genetic susceptibility significantly contributes to the etiology of schizophrenia (33). Although the schizophrenia susceptibility genes are still to be identified, several reports have indicated the location of such gene(s) on chromosome 8 (34) or on the short arm of chromosome 6 (35). Incomplete penetrance and environmental forms of phenocopies still leave room also for gene-environment interactions (36, 37), and some associations of human lymphocyte antigens (HLAs) in schizophrenia have been detected (38–40), suggesting that immune mechanisms may contribute to the etiology of the disease. Viewed from the angle of these processes, the accumulation of macrophages in the CSF during acute schizophrenia reported here may reflect a genetically transformed immune response to (or may be caused by) environmental factors.
Abnormalities of the gross anatomy of the brain are frequently found in schizophrenia. Both autopsy studies and modern brain imaging have revealed a loss of brain substance, in particular from the fronto-occipital areas, and enlargement of the CSF space (13–19). These abnormalities may signify dysregulated brain development in schizophrenia. In addition, there are reports of a correlation between the extent of macroscopic brain abnormalities and the duration of the psychotic disease, which implicates a contribution by some chronic degenerative process in the CNS (41). Macrophage dominance is a frequent finding in CNS injuries (42). Consequently, our detection of an excess of vacuolated macrophages in the CSF of patients with schizophrenia may reflect some organic brain destruction of subchronic or chronic type and relate to the neuroradiologically demonstrated low brain volume in schizophrenia.
Most of the macrophages found in the CSF are considered to be of microglial derivation (43, 44). Microglia, which originate from hematopoietic stem cells (45, 46), constitute the main source for the macrophages accumulating in lesions of the CNS. In addition to their phagocytic capacity, microglial cells contribute to the immune network in the CNS by expressing HLA-DR molecules (47) and acting as antigen-presenting cells (48). The absence of neutrophil recruitment and the delay in the increase in macrophage or microglial cells show that the CNS differs from other sites in the body with regard to the kinetics and nature of the myelomonocytic cell responses (49). Coculturing of microglia with T lymphocytes results in clustering of T cells around the microglia and initiation of mixed lymphocyte reaction (50), and activated T lymphocytes induce the cells of the macrophage lineage to produce pro-inflammatory cytokines (51). Moreover, the CNS mononuclear cells express receptors for neurotransmitters and may therefore functionally bridge the CNS and the immune system (52, 53). The macrophage dominance in the CSF of psychotic patients detected in this study may reflect activation or mobilization of the microglial cells, or both, and may also link the previous reports on T lymphocyte deviations and cytokine aberrations in schizophrenia.
Given that 5-HT has been found to act as a modifier of dopamine responses (54) and as an activator of macrophages (55), it is tempting to speculate that abnormal monoamine metabolism in acute schizophrenia may contribute to the mobilization or activation of microglia leading to the relative macrophage dominance in the CSF. Our finding of neuroleptic-induced normalization of the CSF cytology in many patients lends support to this hypothesis.
A macrophage dominance over lymphocytes, as in the CSF of the adult schizophrenic patients in this study, is also typical in the CSF of newborn infants, and normally the cell profile gradually transforms with age into that of normal adults (i.e., lymphocyte dominance of 60%–80%) (27). This phenomenon may have bearing on the neurodevelopmental theories of schizophrenia (2, 3), especially because programmed cell death (apoptosis) and axonal pruning are thought to be essential actions in neurodevelopmental sequences, are substantially affected by macrophages and microglia, and are influenced by both genetic and environmental factors (56).
Macrophages have numerous effects on the CNS mediated by cytokines and neurotoxins (56), and further CSF analysis of, for example, pro- and anti-inflammatory cytokines and excitatory amino acids will be needed to further clarify the more detailed role of macrophages and microglia in the pathogenesis of schizophrenia.
Presented in part at the workshop Critical Issues in the Treatment of Schizophrenia, March 10–12, 1995, Florence, Italy. Received Oct. 27, 1997; revision received April 30, 1999; accepted May 7, 1999. From the Departments of Psychiatry and Pathology, Helsinki University; and Hesperia Hospital, Helsinki. Address reprint requests to Dr. Nikkil⪠Section of Clinical Neurosciences, The Finnish Institute of Occupational Health, Topeliuksenkatu 41 a A 00250 Helsinki, Finland; [email protected] (e-mail). Supported by grants from the Academy of Finland, the Sigrid Juselius Foundation, and the Finnish Society of Sciences and Letters. The authors thank Dr. Eero Taskinen for access to the CSF material from comparison subjects and Prof. Ulf G. Ahlfors for permitting collection of clinical material.
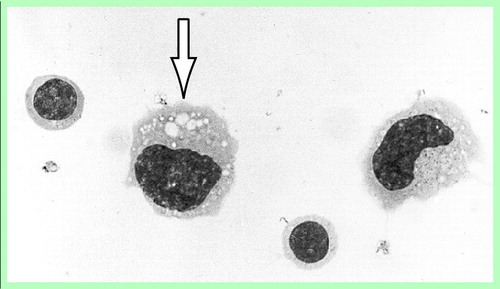
FIGURE 1. Morphological Features of Mononuclear Cells in the CSF of Schizophrenic Patientsa
Two lymphocytes and two macrophages. The arrow indicates a macrophage containing typical cytoplasmic vacuoles.
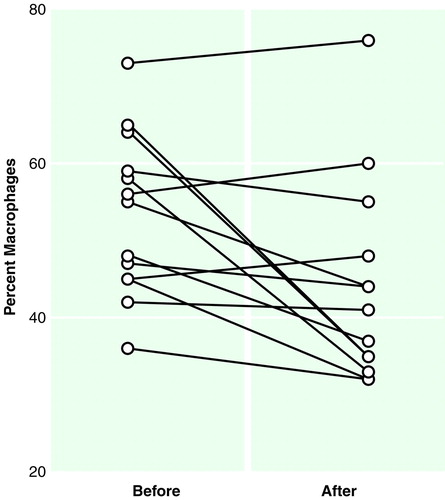
FIGURE 2. Proportion of Macrophages in the CSF of 13 Schizophrenic Patients Before and After Treatment With Typical Neuroleptics
1. Kety SS, Wender PH, Jacobsen B, Ingraham LJ, Jansson L, Faber B, Kinney DK: Mental illness in the biological and adoptive relatives of schizophrenic adoptees: replication of the Copenhagen Study in the rest of Denmark. Arch Gen Psychiatry 1994; 51:442–455Crossref, Medline, Google Scholar
2. Cannon TD, Mednick SA, Parnas J, Schulsinger F, Praestholm J, Vestergaard A: Developmental brain abnormalities in the offspring of schizophrenic mothers, II: structural brain characteristics of schizophrenia and schizotypal personality disorder. Arch Gen Psychiatry 1994; 51:955–962Crossref, Medline, Google Scholar
3. Murray RM, Jones P, O´Callaghan E: Genes, viruses and neurodevelopmental schizophrenia. J Psychiatr Res 1992; 26:225–235Crossref, Medline, Google Scholar
4. Hirata-Hibi M, Hayashi K: The anatomy of the P lymphocyte. Schizophr Res 1993; 8:257–262Crossref, Medline, Google Scholar
5. Muller N, Ackenheil M, Hofschuster E, Mempel W, Eckstein R: Cellular immunity, HLA-class I antigens, and family history of psychiatric disorder in endogenous psychoses. Psychiatry Res 1993; 48:201–217Crossref, Medline, Google Scholar
6. Henneberg AE, Horter S, Ruffert S: Increased prevalence of antibrain antibodies in the sera from schizophrenic patients. Schizophr Res 1994; 14:15–22Crossref, Medline, Google Scholar
7. Nikkilâ, M�K, Ahokas A, Miettinen K, Andersson LC, Rimón R: Abnormal distributions of T-lymphocyte subsets in the cerebrospinal fluid of patients with acute schizophrenia. Schizophr Res 1995; 14:215–221Crossref, Medline, Google Scholar
8. Seeman P, Guan HC, Van-Tol HH: Dopamine D4 receptors elevated in schizophrenia. Nature 1993; 365:441–445Crossref, Medline, Google Scholar
9. Ricci A, Amenta F: Dopamine D5 receptors in human peripheral blood lymphocytes: a radioligand binding study. J Neuroimmunol 1994; 53:1–7Crossref, Medline, Google Scholar
10. Joyce JN, Shane A, Lexow N, Winokur A, Casanova MF, Kleinman JE: Serotonin uptake sites and serotonin receptors are altered in the limbic system of schizophrenics. Neuropsychopharmacology 1993; 8:315–336Crossref, Medline, Google Scholar
11. Laruelle M, Abi-Dargham A, Casanova MF, Toti R, Weinberger DR, Kleinman JE: Selective abnormalities of prefrontal serotonergic receptors in schizophrenia: a postmortem study. Arch Gen Psychiatry 1993; 50:810–818Crossref, Medline, Google Scholar
12. Farde L, Nordstrom AL, Nyberg S, Halldin C, Sedvall G: D1-, D2-, and 5-HT2-receptor occupancy in clozapine-treated patients. J Clin Psychiatry 1994; 55(Sept suppl B):67–69Google Scholar
13. Suddath RL, Casanova MF, Goldberg TE, Daniel DG, Kelsoe JR Jr, Weinberger DR: Temporal lobe pathology in schizophrenia: a quantitative magnetic resonance imaging study. Am J Psychiatry 1989; 146:464–472Link, Google Scholar
14. Suddath RL, Christison GW, Torrey EF, Casanova MF, Weinberger DR: Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N Engl J Med 1990; 322:789–794Crossref, Medline, Google Scholar
15. DeLisi LE, Hoff AL, Schwartz JE, Shields GW, Halthore SN, Gupta SM, Henn FA, Anand AK: Brain morphology in first-episode schizophrenic-like psychotic patients: a quantitative magnetic resonance imaging study. Biol Psychiatry 1991; 29:159–175Crossref, Medline, Google Scholar
16. Zipursky RB, Lim KO, Sullivan EV, Brown BW, Pfefferbaum A: Widespread cerebral gray matter volume deficits in schizophrenia. Arch Gen Psychiatry 1992; 49:195–205Crossref, Medline, Google Scholar
17. Schlaepfer TE, Harris GJ, Tien AY, Peng LW, Lee S, Federman EB, Chase GA, Barta PE, Pearlson GD: Decreased regional cortical gray matter volume in schizophrenia. Am J Psychiatry 1994; 151:842–848Link, Google Scholar
18. Pfefferbaum A, Lim KO, Rosenbloom M, Zipursky RB: Brain magnetic resonance imaging: approaches for investigating schizophrenia. Schizophr Bull 1990; 16:453–476Crossref, Medline, Google Scholar
19. Degreef G, Ashtari M, Bogerts B, Bilder RM, Jody DN, Alvir JM, Lieberman JA: Volumes of ventricular system subdivisions measured from magnetic resonance images in first-episode schizophrenic patients. Arch Gen Psychiatry 1992; 49:531–537Crossref, Medline, Google Scholar
20. McAllister CG, van Kammen DP, Rehn TJ, Miller AL, Gurklis J, Kelley ME, Yao J, Peters JL: Increases in CSF levels of interleukin-2 in schizophrenia: effects of recurrence of psychosis and medication status. Am J Psychiatry 1995; 152:1291–1297Google Scholar
21. Ganguli R, Brar JS, Chengappa KR, DeLeo M, Yang ZW, Shurin G, Rabin BS: Mitogen-stimulated interleukin-2 production in never-medicated, first-episode schizophrenic patients: the influence of age at onset and negative symptoms. Arch Gen Psychiatry 1995; 52:668–672Crossref, Medline, Google Scholar
22. Ganguli R, Yang Z, Shurin G, Chengappa KN, Brar JS, Gubbi AV, Rabin BS: Serum interleukin-6 concentration in schizophrenia: elevation associated with duration of illness. Psychiatry Res 1994; 51:1–10Crossref, Medline, Google Scholar
23. Naudin J, Capo C, Giusano B, Mege JL, Azorin JM: A differential role for interleukin-6 and tumor necrosis factor-alpha in schizophrenia? Schizophr Res 1997; 26:227–233Google Scholar
24. Venezie RD, Toews AD, Morell P: Macrophage recruitment in different models of nerve injury: lysozyme as a marker for active phagocytosis. J Neurosci Res 1995; 40:99–107Crossref, Medline, Google Scholar
25. Lotan M, Schwartz M: Cross talk between the immune system and the nervous system in response to injury: implications for regeneration. FASEB J 1994; 8:1026–1033Google Scholar
26. Troost D, Claessen N, van den Oord JJ, Swaab DF, de Jong JM: Neuronophagia in the motor cortex in amyotrophic lateral sclerosis. Neuropathol Appl Neurobiol 1993; 19:390–397Crossref, Medline, Google Scholar
27. Oehmichen M: Cerebrospinal Fluid Cytology. Stuttgart, Germany, G Thieme, 1976Google Scholar
28. Cook JD, Brooks BR: Lymphocyte subpopulations in human cerebrospinal fluid, in Neurobiology of Cerebrospinal Fluid. Edited by Wood JH. New York, Plenum, 1980, pp 507–523Google Scholar
29. Taskinen E: Cerebrospinal Fluid Cells and Proteins in Neurologic Diseases With Known or Suspected Immunoactivation of the Central Nervous System (thesis). Helsinki, University of Helsinki, Transplantation Laboratory and Department of Neurology, 1983Google Scholar
30. M�K: Involvement of the Nervous System in the Immunobiology of Myasthenia Gravis (thesis). Helsinki, University of Helsinki, Departments of Neurology and Pathology and Transplantation Laboratory, 1990Google Scholar
31. Koelmel HW: Atlas of Cerebrospinal Fluid Cells, 2nd ed. New York, Springer-Verlag, 1977Google Scholar
32. M�KMI, Elovaara I, Haltia M: Cerebrospinal fluid cytology in early HIV-1 infection, in An Atlas of the Neuropathology of HIV Infection. Edited by Gray F. New York, Oxford University Press, 1993, pp 239–243Google Scholar
33. Vallada HP, Kunugi H: An overview of schizophrenia genetic research presented at the 1995 World Congress on Psychiatric Genetics, Cardiff. Schizophr Res 1996; 19:87–92Crossref, Medline, Google Scholar
34. Kendler KS, MacLean CJ, O’Neill FA, Burke J, Murphy B, Duke F, Shinkwin R, Easter SM, Webb BT, Zhang J, Walsh D, Straub RE: Evidence for a schizophrenia vulnerability locus on chromosome 8p in the Irish Study of High-Density Schizophrenia Families. Am J Psychiatry 1996; 153:1534–1540Google Scholar
35. Turecki G, Rouleau GA, Joober R, Mari J, Morgan K: Schizophrenia and chromosome 6p. Am J Med Genet 1997; 74:195–198Crossref, Medline, Google Scholar
36. Dawson E, Murray R: A gene at 6p? schizophrenia. Curr Biol 1996; 6:268–271Crossref, Medline, Google Scholar
37. Yolken RH, Torrey EF: Viruses as etiologic agents of schizophrenia, in Advances in Biological Psychiatry, vol 18. Edited by Ebert D, Ebmeier WP, Kaschka WP, Rechlin T. Basel, Switzerland, Karger, 1997, pp 1–12Google Scholar
38. Zamani MG, De Hert M, Spaepen M, Hermans M, Marynen P, Cassiman JJ, Peuskens J: Study of the possible association of HLA class II, CD4, and CD3 polymorphisms with schizophrenia. Am J Med Genet 1994; 54:372–377Crossref, Medline, Google Scholar
39. Blackwood DH, Muir WJ, Stephenson A, Wentzel J, Ad’hiah A, Walker MJ, Papiha SS, St Clair DM, Roberts DF: Reduced expression of HLA-B35 in schizophrenia. Psychiatr Genet 1996; 6:51–59Crossref, Medline, Google Scholar
40. Wright P, Donaldson PT, Underhill JA, Choudhuri K, Doherty DG, Murray RM: Genetic association of the HLA DRB1 gene locus on chromosome 6p21.3 with schizophrenia. Am J Psychiatry 1996; 153:1530–1533Google Scholar
41. DeLisi LE, Sakuma M, Tew W, Kushner M, Hoff AL, Grimson R: Schizophrenia as a chronic active brain process: a study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry Res 1997; 74:129–140Crossref, Medline, Google Scholar
42. Thomas WE: Brain macrophages: evaluation of microglia and their functions. Brain Res Brain Res Rev 1992; 17:61–74Crossref, Medline, Google Scholar
43. De Groot CJ, Huppes W, Sminia T, Kraal G, Dijkstra CD: Determination of the origin and nature of brain macrophages and microglial cells in mouse central nervous system, using non-radioactive in situ hybridization and immunoperoxidase techniques. Glia 1992; 6:301–309Crossref, Medline, Google Scholar
44. Kettenmann H, Banati R, Walz W: Electrophysiological behavior of microglia. Glia 1993; 7:93–101Crossref, Medline, Google Scholar
45. Streit WJ, Graeber MB, Kreutzberg GW: Functional plasticity of microglia: a review. Glia 1988; 1:301–307Crossref, Medline, Google Scholar
46. Peress NS, Fleit HB, Perillo E, Kuljis R, Pezzullo C: Identification of Fc gamma RI, II and III on normal human brain ramified microglia and on microglia in senile plaques in Alzheimer’s disease. J Neuroimmunol 1993; 48:71–79Crossref, Medline, Google Scholar
47. Gehrmann J, Banati RB, Kreutzberg GW: Microglia in the immune surveillance of the brain: human microglia constitutively express HLA-DR molecules. J Neuroimmunol 1993; 48:189–198Crossref, Medline, Google Scholar
48. Graeber MB, Streit WJ: Microglia: immune network in the CNS. Brain Pathol 1990; 1:2–5Crossref, Medline, Google Scholar
49. Andersson PB, Perry VH, Gordon S: The kinetics and morphological characteristics of the macrophage-microglial response to kainic acid-induced neuronal degeneration. Neuroscience 1991; 42:201–214Crossref, Medline, Google Scholar
50. Ulvestad E, Williams K, Bjerkvig R, Tiekotter K, Antel J, Matre R: Human microglial cells have phenotypic and functional characteristics in common with both macrophages and dendritic antigen-presenting cells. J Leukoc Biol 1994; 56:732–740Medline, Google Scholar
51. Chabot S, Williams G, Yong VW: Microglial production of TNF-alpha is induced by activated T-lymphocytes: involvement of VLA-4 and inhibition by interferonbeta-1B. J Clin Invest 1997; 100:604–612Crossref, Medline, Google Scholar
52. McGillis JP, Mitsuhashi M, Payan DG: Immunomodulation by tachykinin neuropeptides. Ann NY Acad Sci 1990; 594:85–94Crossref, Medline, Google Scholar
53. Martin FC, Anton PA, Gornbein JA, Shanahan F, Merrill JE: Production of interleukin-1 by microglia in response to substance P: role for a non-classical NK-1 receptor. J Neuroimmunol 1993; 42:53–60Crossref, Medline, Google Scholar
54. Hagan RM, Kilpatrick GJ, Tyers MB: Interactions between 5-HT3 receptors and cerebral dopamine function: implications for the treatment of schizophrenia and psychoactive substance abuse. Psychopharmacology (Berl) 1993; 112(suppl 1):68–75Google Scholar
55. Young MR, Matthews JP: Serotonin regulation of T-cell subpopulations and of macrophage accessory function. Immunology 1995; 84:148–152Medline, Google Scholar
56. Fricchione GL, Bilfinger TV, Stefano GB: The macrophage and neuropsychiatric disorders. Neuropsychiatry Neuropsychol Behav Neurol 1996; 9:16–29Google Scholar



