Serotonin 5-HT2 Receptors in Schizophrenia: A PET Study Using [18F]Setoperone in Neuroleptic-Naive Patients and Normal Subjects
Abstract
OBJECTIVE: Several postmortem studies have reported a decreased density of serotonin 5-HT2 receptors in the prefrontal cortex in schizophrenia. The purpose of this study was to investigate this in patients with schizophrenia by means of [18F]setoperone and positron emission tomography (PET) imaging. METHOD: Thirteen neuroleptic-free patients with schizophrenia, 10 of whom were also neuroleptic-naive, were compared with a group of 26 normal subjects in the same age range. The density of 5-HT2 receptors was assessed with the use of [18F]setoperone and PET in standardized cortical regions of interest. RESULTS: Increasing age was associated with similar declines in 5-HT2 receptors in all cortical regions in the patient group and in the normal comparison group. After control for the effect of age, there was no statistically significant difference between the patients and the comparison subjects in 5-HT2 receptor density in any of the cortical regions. CONCLUSIONS: This study failed to find the decrease in 5-HT2 receptors reported in postmortem studies of schizophrenia. The study had the power to detect a decrease of 25% or more in 5-HT2 receptors, which was anticipated on the basis of the previous postmortem studies. Thus, a primary serotonergic abnormality in schizophrenia, if one exists, is either small or unlikely to be at the level of the 5-HT2 receptors. This finding does not rule out a therapeutic role for 5-HT2 antagonists in schizophrenia, but it does suggest that the therapeutic contribution is likely to be an indirect one.
The serotonergic hypothesis of schizophrenia preceded the better-known dopaminergic hypothesis. Following Gaddum’s demonstration in 1953 that LSD, a hallucinogenic drug, had affinity for serotonin receptors, several lines of evidence suggested a role for serotonin in the pathophysiology of schizophrenia (1–4). However, the development of effective antipsychotic drugs that were antagonists at dopamine receptors led to a greater focus on and investigation of the dopamine system (5). With the success of clozapine as an “atypical” antipsychotic (6), interest in the role of serotonin in schizophrenia has been renewed.
The serotonergic hypothesis is best viewed as complementary to the dopaminergic hypothesis, rather than as an alternative to it, since these systems are anatomically connected and functionally interactive (7, 8). Several authors have proposed that the atypical antipsychotics derive their unique efficacy at least partly from their dual serotonin-dopamine antagonism, exploiting the neurochemical interaction between these two systems (2, 4, 7, 9, 10). Despite the recent interest in the role of serotonin 5-HT2 receptors, the nature of the 5-HT2 abnormality in schizophrenia, if any, is not known. Until recently, the means to specifically measure 5-HT2 receptor density in the brains of living patients with schizophrenia were unavailable. Therefore, our current knowledge is derived mainly from studies of 5-HT2 receptors in postmortem brain tissue and on platelet membranes (11, 12). The 5-HT system in platelets is developmentally related to the 5-HT system in the brain, but the exact relationship (and, hence, the correlation in disease states) is not completely understood (4).
Postmortem techniques have demonstrated the presence of 5-HT2 receptors throughout the cortex and in subcortical structures in normal human brains (13–16). Postmortem investigations of cortical 5-HT2 receptors in schizophrenia are summarized in table 1.
A review of the literature on postmortem findings suggests a decrease in 5-HT2 receptors in schizophrenia, but this is not conclusive. While some studies suggest a decrease (17, 20– 22, 25, 26), others do not confirm this (18, 19, 23, 24). The reported decrease in Bmax (the number of 5-HT2 receptors) is in the range of 25%–55 % (17, 20–22, 25, 26). While there is some inconsistency regarding studies of Bmax, there is unanimity regarding the affinity (Kd) of these receptors: no study has reported a significant alteration in the Kd of the receptors in patients with schizophrenia (17–26). The previous studies focused mainly on the prefrontal cortex; even so, the different studies focused on different subregions within it. Few studies have investigated other cortical regions (Laruelle et al. [22] reported on the occipital cortex, while Joyce et al. [23] reported on the limbic regions), even though 5-HT2 receptors are uniformly distributed throughout the cortex. Postmortem studies are often confounded by issues of inaccurate diagnosis, different causes of death, different postmortem intervals before tissue processing for analysis, and the effects of concurrent illnesses and medications at the time of death. Some of the studies attempted to address the question of the effect of exposure to neuroleptics, with differing results (18, 20, 22, 25). To address these shortcomings, we studied 5-HT2 receptors in neuroleptic-free patients and a group of normal comparison subjects, using [18F]setoperone and positron emission tomography (PET) imaging (27–30).
Setoperone binds to 5-HT2 receptors with high affinity (Ki∼1 nM). It is relatively selective for the 5-HT2A subtype, since its affinity for the dopamine D2 receptor is an order of magnitude lower (Ki∼10–25 nM), and its affinity for the 5-HT2C receptor is lower still (Ki∼50–80 nM) (31). It has been shown to bind specifically to 5-HT2 receptors in both in vitro and ex vivo preparations. When radiolabeled with fluorine-18, setoperone has several features that make it well-suited for PET studies of 5-HT2 receptors. It rapidly equilibrates across the blood-brain barrier, binds reversibly, and has no metabolites that cross the blood-brain barrier (32). In PET studies, the cortical signal can be blocked by the competitive antagonist ketanserin and is unaffected by sulpiride, a D2 blocker, suggesting that [18F]setoperone binds specifically to 5-HT2A receptors (27, 28, 30). Furthermore, the cerebellum is a region almost devoid of 5-HT2 receptors in postmortem studies (13, 15), and accordingly, the [18F]setoperone signal from the cerebellum is not affected by 5-HT2 antagonists. Since the cerebellum represents free ligand and nonspecific [18F]setoperone binding (30), using it as a reference region enables one to obtain semiquantitative estimates of Bmax/Kd, indexes of 5-HT2 binding potential, without arterial puncture or kinetic modeling, making it suitable for routine clinical studies (33). With the use of these methods, it has been shown that [18F]setoperone is sensitive to the effects of age on 5-HT2 receptors, shows robust effects of illnesses such as Alzheimer’s dementia (34), and is parametrically sensitive to the effects of antipsychotics that bind to 5-HT2 receptors (35).
An alternative ligand for the study of 5-HT2 receptors is [18F]altanserin. [18F]Altanserin’s specificity for subcortical 5-HT2 receptors is slightly higher than that of [18F]setoperone, since setoperone has moderate affinity for D2 receptors (36). However, because D2 receptors are undetectable in the cortex, the effective specificity for these two ligands is equal for the study of cortical 5-HT2 receptors (37). Another radioligand that is used is N1-([11C]methyl)-2-Br-LSD, which is less selective than [18F]setoperone or [18F]altanserin (37, 38).
At the outset we were interested in two issues. First, on the basis of postmortem data, we hypothesized that the binding potential of [18F]setoperone for 5-HT2 receptors would be decreased in the prefrontal cortex in schizophrenic patients as compared with healthy community volunteers. Second, we wanted to explore any differences in 5-HT2 receptors in nonfrontal regions, since this issue has never been systematically addressed in any previous study.
METHOD
The study was approved by the Human Subjects Review Committee of the University of Toronto. The subjects participated after receiving information regarding the study and providing written consent. Thirteen patients (10 male and three female), whose mean age was 31.1 years (SD=6.9, range=21–43), were included in the study. A 14th patient (male, aged 46 years) participated in the study, but his data were excluded from the analysis because of the poor quality of his PET scan. All patients had a DSM-IV diagnosis of schizophrenia. Patients were assessed by a chart review, discussion with the treating physician, and a clinical interview in which the Structured Clinical Interview for DSM-IV Axis I Disorders (39) was used. The current symptomatic status of the patients was assessed with the Positive and Negative Syndrome Scale (40).
The patients were recruited from a university-affiliated psychiatric hospital receiving referrals from a large urban region. The patients’ mean duration of illness, from the first onset of psychotic symptoms, was 4.7 years (SD=3.7 years, range=3 weeks to 10 years) at the time of their scans. Two patients had been ill for less than 6 months at the time of their scans and were therefore provisionally diagnosed as having schizophreniform disorder. In both of these cases, the diagnosis was revised to schizophrenia in subsequent follow-up. Ten patients were neuroleptic-naive, and three were neuroleptic-free. The neuroleptic-free patients had the following neuroleptic-free intervals and prior cumulative exposure to neuroleptics: 7 weeks free with 15 months’ exposure, 4 months free with 4 years’ exposure, and 10 months free with 1 month’s exposure. The previous exposures were accompanied by poor compliance in all cases. Only one patient had received a depot neuroleptic, and that was more than 3 years before the study.
The normal comparison group consisted of 26 subjects (11 male and 15 female) whose mean age was 31.3 years (SD=6.7, range=19–43). These subjects were healthy volunteers recruited from the community by advertisement. They were stratified over the age range of interest (eight to 10 subjects for each decade) to provide a reliable estimate of age effects. They were screened with the Structured Clinical Interview for DSM-III-R—Non-Patient Edition (41) to ensure that they had no history of psychiatric disorder.
Neither patients nor comparison subjects were currently using any psychotropic medications (with the exception of benzodiazepines for the patients), alcohol, or street drugs. None of the subjects had a history of alcohol or drug dependence, significant head injury, major neurological disorder, or other serious medical illness.
Radiosynthesis and PET Imaging
[18F]Setoperone was synthesized by a method described previously (33). PET scans were acquired with the use of a GEMS PC2048-15B head-dedicated PET camera (GE Medical Systems, Milwaukee). Subjects were fitted with a thermoplastic mask to limit head movement. A transmission scan was acquired with a germanium-68 rotating pin source to measure attenuation, prior to a bolus injection of [18F]setoperone (injected dose range=4.24–5.57 mCi; specific activity range=360–6210 mCi/µmol). PET data were acquired over the next 90 minutes and reconstructed into 22 time frames (five 1-minute frames and then 17 5-minute frames), with 15 6.5-mm-thick axial slices, with the use of a Hanning filter of 5-mm full width at half maximum. Magnetic resonance imaging (MRI) scans were acquired separately with a GE Signa 1.5-T scanner, spin-echo sequence, in an axial orientation, yielding a whole-brain image with 42 3-mm-thick slices. The MRI scans were coregistered with the PET scans by means of the surface-matching algorithm implemented in ANALYZE (CNS Software, Rochester, Minn.).
Data Analysis
Cortical regions of interest were manually traced onto the axial PET images, with the use of Alice 3.0 image analysis software (Hayden Image Processing Group, Denver) implemented on a personal computer-based Windows NT platform, according to a set of conservative anatomical rules based on neuroanatomical landmarks and guided by a standard neuroanatomical atlas (42). The coregistered MRI slices were used as a visual reference to provide more precise anatomical definition in the drawing of the regions of interest on the PET images. Eight cortical regions of interest were defined: the left and right prefrontal, temporal, parietal, and occipital cortices. A single region of interest was defined that encompassed both hemispheres of the cerebellum. The prefrontal cortical regions of interest were defined on five contiguous PET slices for each hemisphere; the temporal, parietal, and occipital regions of interest were defined on three contiguous slices in each hemisphere; and the cerebellar regions of interest were defined on two contiguous slices. These multiple slices were used in order to reduce partial volume effects. All of the slices within a given region of interest were combined for the analysis. All of the regions of interest were drawn by one of us (R.L.), who was blind to the identity of the subjects.
For each cortical region of interest, a time-activity curve was obtained, reflecting the fluorine-18 activity in the region of interest. The ratio of fluorine-18 activity in the specific cortical region (S) to that in the cerebellum (C) (i.e., S/C–1) at 65–90 minutes was used as an index of 5-HT2 receptors. The cerebellum was used as a reference region, serving as a measure of free and nonspecific binding of [18F]setoperone. The time window of 65–90 minutes was chosen because the S/C–1 ratio was stable and showed no statistical change during this window (33). Theoretically, it can be shown that under such conditions and assumptions, the S/C–1 ratio is linearly proportional to the Bmax/Kd of [18F]setoperone for 5-HT2 receptors. In addition, Petit-Taboue et al. (43) have shown empirically that the ratio during this time window is highly correlated (r values=0.91–0.97) with the values of Bmax/Kd as obtained from compartmental kinetic modeling. We call this semiquantitative index “5-HT2R” in further discussions to distinguish it from fully quantitative values of Bmax or Kd.
Before this study we had established that this imaging protocol can be repeated in the same individual with high test-retest reliability (intraclass correlation coefficients=0.96–0.98) (33). Also, we had standardized the technique of data analysis and established high intrarater and interrater reliability (intraclass correlation coefficient, type III: intrarater r>0.98 for author R.L., and interrater r>0.95 for authors R.L., S.K., and C.J.) (33).
Previous studies (30, 34, 44–46) had shown decreases in 5-HT2 receptors with age, a finding that we confirmed. Therefore, it was planned that age would be a covariate in our analysis. We used two statistical strategies. First, since we had an a priori hypothesis regarding a decrease in these receptors in the prefrontal cortex of patients with schizophrenia, we compared the 5-HT2R for the two groups by using an analysis of covariance (ANCOVA) with age as a covariate to assess the main effect of diagnosis (i.e., schizophrenia) and to investigate any significant interactions between age and diagnosis. Second, to investigate the 5-HT2R in other brain regions, we undertook a multivariate analysis of covariance (MANCOVA) with the four major brain regions (frontal, temporal, parietal, and occipital) as dependent variables and age as a covariate. Statistical analyses were done with SPSS version 7.0 for Windows (SPSS Inc., Chicago).
RESULTS
The values of 5-HT2R for the right and for the left regions were not statistically differentiable and were highly correlated for all of the cortical regions (Pearson’s r=0.98–0.99); they were therefore averaged for further analysis. There was no significant effect of sex in the group of normal comparison subjects (ANCOVA: F=1.64, df=1, 22, p=0.21). There were too few women in the patient group (N=3) to permit a valid comparison of the effect of sex on 5-HT2R in the patients. To rule out any bias due to too few female patients, we analyzed the results in the male subgroup only (i.e., the 11 male comparison subjects and the 10 male patients). The results in the male subgroup were no different from those for the complete study group; therefore, we report here a pooled analysis of male and female subjects, comparison subjects as well as patients.
There was no significant difference in activity of [18F]setoperone in the cerebellum (i.e., the denominator “C” in the ratio S/C–1, used to derive the 5-HT2R) between patients and comparison subjects (F=0.60, df=1, 35, p=0.45), indicating that there was no significant difference between the groups in free ligand and nonspecific binding; a difference here could have confounded the interpretation of 5-HT2R and therefore was important to evaluate. Furthermore, there was no significant difference between the groups in the volumes of any of the regions of interest, suggesting that a difference in determination of the regions of interest was not confounding the results.
As expected, for the study group as a whole (patients and comparison subjects), there was a substantial decrease in 5-HT2R with age (Pearson’s r=0.80; F=67.42, df=1, 37, p<0.001) (figure 1). With age as a covariate, we found no significant effect of diagnosis on 5-HT2R (i.e., no significant difference in 5-HT2R between patients and comparison subjects) in the prefrontal region. The mean prefrontal 5-HT2R was=1.66 (SD=0.50; age-adjusted mean=1.65) for the patient group and 1.85 (SD=0.66; age-adjusted mean=1.85) for the comparison group (ANCOVA: F=1.45, df=1, 35, p=0.24) (figure 1). While age had a significant effect on 5-HT2R in both patients and comparison subjects, the age-associated decline was not significantly different between the two groups (ANCOVA: F=0.76, df=1, 35, p=0.39).
The second important question was the effect of schizophrenia on 5-HT2 receptors in all of the brain regions. Since this was an exploratory question, and the regions are highly interrelated, the appropriate method to address this question is a multivariate analysis. A MANCOVA showed an effect of age but no effect of illness. With the four cortical regions as dependent variables and age as a covariate, there was no effect of illness (Pillai’s trace statistic=0.08, and Wilks’s lambda=0.92; F=0.69, df=4, 32, p=0.60). Age itself had a very significant multivariate effect (Pillai’s trace statistic=0.68, and Wilks’s lambda=0.32; F=16.69, df=4, 32, p<0.001), but there was no interaction between the effects of age and illness (Pillai’s trace statistic=0.06, and Wilks’s lambda=0.94; F=0.54, df=4, 32, p=0.71). Quantitatively, the 5-HT2R results were highly correlated across the brain (Pearson’s r values=0.95–0.98), and the values in the different brain regions were as follows: temporal region—patients’ mean=1.79 (SD=0.52; age-adjusted mean=1.78), and comparison subjects’ mean=1.92 (SD=0.68; age-adjusted mean=1.93); parietal region—patients’ mean=1.66 (SD=0.49; age-adjusted mean=1.65), and comparison subjects’ mean=1.80 (SD=0.63; age-adjusted mean=1.80); occipital region—patients’ mean=1.75 (SD=0.41; age-adjusted mean=1.75), and comparison subjects’ mean=1.92 (SD=0.59; age-adjusted mean=1.92).
We found no significant relationship between Positive and Negative Syndrome Scale total score, positive or negative subscale scores, and 5-HT2R for any of the regions of interest, suggesting that differences in 5-HT2R are not associated with profile of symptoms. Finally, three of our patients had received neuroleptics, while the other 10 were neuroleptic-naive. Repeating the MANCOVA in the neuroleptic-naive group, we still found no significant effect of illness (Pillai’s trace statistic=0.06, and Wilks’s lambda=0.94; F=0.45, df=4, 29, p=0.78).
DISCUSSION
This study constitutes the first systematic effort to assess 5-HT2 receptors in schizophrenic patients with the use of neuroreceptor imaging. With [18F]setoperone and PET, the study did not find a significant decrease in 5-HT2 receptors in patients with schizophrenia. These findings are at odds with some of the previous postmortem studies. We now discuss the limitations of postmortem studies, the limitations of our own methods, and the implications of these findings for understanding the role of 5-HT in the pathophysiology and therapeutics of schizophrenia.
Studying neuroreceptors in living patients offers several advantages over postmortem studies. In vitro postmortem studies face confounding variables that may explain some of the inconsistencies in findings between studies (4, 23, 25, 26). Technical methods vary between laboratories. Postmortem changes in the brain may affect neuroreceptors, depending on the time elapsed between death and preparation of the specimen. Clinical data about the subjects are usually reconstructed retrospectively, and it is difficult to control for clinical variables, especially exposure to neuroleptics before death, which could affect 5-HT2 receptor binding. None of the postmortem studies included neuroleptic-naive subjects, and the type and degree of cumulative exposure varied greatly. Finally, there are no true “normal” control subjects in postmortem studies, since all subjects have died through some disease process or trauma, factors which could affect 5-HT2 receptor assessment. The combination of these factors may explain the inconsistency in the literature on postmortem studies and may also explain why our findings are at variance with the postmortem data.
Our study is not without its limitations. We used an index of 5-HT2 receptors that reflects the ratio of receptor density to receptor affinity (i.e., Bmax/Kd) but cannot provide independent measures of either Bmax or Kd. A separate measurement of Bmax would be theoretically desirable. However, it should be pointed out that none of the previous postmortem studies has suggested any change in Kd in schizophrenia. Thus, it is very unlikely that Kd could have obscured a change in Bmax. Furthermore, a change in Kd would hide a change in Bmax only if both Bmax and Kd changed simultaneously, in the same numerical direction, by exactly the same extent—a rather unlikely scenario.
It is important to note that while our data suggest no change in receptor number, they do not address receptor function. The study does not tell us to what extent 5-HT2 receptors have the same or different functional properties in the two groups. It is of interest that studies using pharmacological challenges which probe the 5-HT system (e.g., the 5-HT releasing agent and reuptake inhibitor fenfluramine or the 5-HT agonist m-chlorophenylpiperazine) have pointed to abnormal 5-HT function in schizophrenia (4, 47–52) or at least in that subgroup of patients who benefit from treatment with 5-HT2 antagonist neuroleptics such as clozapine (48, 53, 54). However, the results of these studies are not consistent. Furthermore, an alteration in the neuroendocrine response to a 5-HT2 challenge does not localize the deficit at the level of the 5-HT2 receptor, for it can equally plausibly represent postreceptor or primary endocrine system alterations.
Our method of region of interest analysis also has its limitations. While we analyzed more brain regions and more brain volumes than any of the previous postmortem studies, we did not analyze the entire cortex. By choosing five slices for the prefrontal cortex and three for the temporal cortex, and so on, we are reporting on representative regions. Such an analysis lumps together smaller regions within the region of interest, and it is conceivable that if there are very localized changes in schizophrenia (i.e., if the 5-HT2 decreases are limited to a very circumscribed region, for example, just Brodmann’s area 9), we may have missed them. However, there is little uniformity in the postmortem findings that could have focused our analysis further. A voxel-by-voxel analysis of the entire brain space would have been a more sensitive approach for exploring circumscribed changes. While such approaches have been used for comparisons of regional blood flow and are in principle possible for receptor studies, they have not yet been standardized for these purposes. Examining this issue by using a voxel-by-voxel analysis remains a project for the future.
Finally, there is no logical way to “prove” the null hypothesis. The best one can do is to state the certainty with which one can reject a null hypothesis. The postmortem studies showed changes in receptor density of approximately 25%–55%. On the basis of these numbers, we estimated that a study of 12 patients would give us sufficient power to rule out a 25% difference. A post hoc power analysis confirmed our initial assumption. Since there was no difference in the effect of age in the two study groups, and age-corrected variance was similar across the two groups, a post hoc analysis suggests that with 13 patients we had a more than 80% power to detect a 25% decrease at a significance level of p<0.05 (55). Could a decrease smaller than 25% exist? Our data do in fact show a nonsignificant trend in that direction. The patients had a numerical decrease in the range of 10%, but such a change did not reach significance in our study. In fact, if one wanted to design a study to detect a 10% change with reasonable certainty (i.e., greater than 80% power to detect a change at p<0.05), one would need 60–70 patients, a considerable task for any single research site. Thus, while we can rule out a decrease of 25% or greater with reasonable certainty, a smaller change may exist.
The results from this study have significant implications, since it is the first systematic PET study of 5-HT2 receptor density in untreated patients with schizophrenia. The serotonergic hypothesis of schizophrenia holds that 5-HT is involved in the neurochemistry of psychosis. Serotonergic agents such as LSD can induce hallucinations. The literature reviewed in this article, based on postmortem studies, suggests that decreased numbers of 5-HT2 receptors may be the basis of a serotonergic abnormality in schizophrenia. If the negative results from the present study in living patients are correct, then this suggests that a serotonergic abnormality, if it exists, is not at the level of 5-HT2 receptors.
Targeting the 5-HT2 receptor has been an important factor in the design of the new generation of atypical antipsychotics, all of which possess 5-HT2 antagonist properties. If there is no abnormality of 5-HT2 receptor density in schizophrenia and yet 5-HT2 antagonism is held to confer some unique therapeutic efficacy, the efficacy of 5-HT2 antagonist drugs must be produced by some indirect mechanism. Such a discrepancy between lack of receptor involvement in disease etiology and a value of that receptor in therapeutics is not new to schizophrenia at all. While it has been difficult to confirm an alteration in dopamine D2 receptors in schizophrenia, there is no denying a role of these receptors in therapeutics (56). Such could be the case for 5-HT2 receptors. In fact, it has been postulated that 5-HT2 antagonists may exert their effects through a modulation of the dopaminergic system (7). Thus, while our data argue against a significant decrease in 5-HT2 receptors in patients with schizophrenia, they do not rule out the role of 5-HT2 receptors in the therapeutics of schizophrenia.
Our study also shows a dramatic effect of age on 5-HT2 receptors. In fact, the 5-HT2 receptor index decreased by about 2%–3% per year in our subjects. Such a decline has been demonstrated by others previously (30, 34, 44– 46). This decline has potentially important consequences for medication dosage. All current atypical antipsychotics block 5-HT2 receptors. It is conceivable that as one ages, the receptor reserve declines, and older patients may be more sensitive to the effects of 5-HT2 receptor blockers. Most clinical trials are focused on adult populations in their 30s or so; thus, there are few systematic data in older age groups. Our finding should alert clinicians to the possibility that the elderly may be particularly sensitive to these medications.
In summary, we failed to find a decrease in 5-HT2 receptors in the prefrontal cortex or other brain regions in schizophrenia. Our results are in contrast to those of the majority of postmortem studies, which show a decrease of 25%–55%. It is not logically possible to “prove” that there is no difference, but our data suggest that if there is any decrease in 5-HT2 receptors at all, the magnitude of decrease is small and may be localized to a small brain region. The results do not rule out the involvement of 5-HT2 receptors in the therapeutics of schizophrenia but suggest that any such benefits are obtained through indirect mechanisms. It will be important to replicate these results with other ligands and with greater statistical power. Future research will also need to be directed at other 5-HT receptor subtypes (in particular the 5-HT1A subtype) and at other levels (e.g., intracellular second messengers) within the serotonergic pathways.
Presented in part at the annual meeting of the Society of Biological Psychiatry, San Diego, May 14–18, 1997. Received Dec. 2, 1997; revision received May 28, 1998; accepted June 10, 1998. From The Clarke Institute of Psychiatry, Department of Psychiatry, University of Toronto. Address reprint requests to Dr. Kapur, PET Centre, The Clarke Institute of Psychiatry, 250 College St., Toronto, ON, Canada M5T 1R8; [email protected] (e-mail). Supported by an award from the Ontario Mental Health Foundation to Dr. Lewis and by a Clinician Scientist Award from the Medical Research Council of Canada to Dr. Kapur. The authors thank Terry Bell, Kevin Cheung, Steven Dobbin, Doug Hussey, and Erin Toole for their technical assistance; Dr. Paul Roy for his assistance with patient recruitment; and Janssen-Cilag France for providing the setoperone precursor.
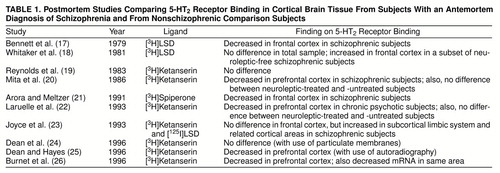 |
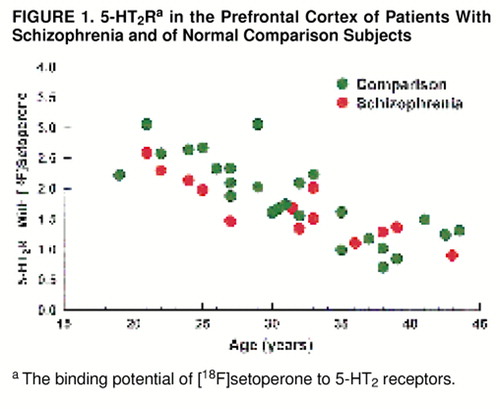
FIGURE 1. 5-HT2Ra in the Prefrontal Cortex of Patients With Schizophrenia and of Normal Comparison Subjects
aThe binding potential of [18F]setoperone to 5-HT2 receptors.
1. Breier A: Serotonin, schizophrenia and antipsychotic drug action. Schizophr Res 1995; 14:187–202Crossref, Medline, Google Scholar
2. Hattunen M: The evolution of the serotonin-dopamine antagonist concept. J Clin Psychopharmacol 1995; 15:4S–10SCrossref, Google Scholar
3. Iqbal N, van Praag HM: The role of serotonin in schizophrenia. Eur Neuropsychopharmacol 1995; 5:11–23Crossref, Medline, Google Scholar
4. Bleich A, Brown S-L, van Praag HM: A serotonergic theory of schizophrenia, in The Role of Serotonin in Psychiatric Disorders. Edited by Brown S-L, van Praag HM. New York, Brunner/Mazel, 1991, pp 183–214Google Scholar
5. Seeman P, Lee T, Chau-Wong M, Wong K: Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature 1976; 261:717–719Crossref, Medline, Google Scholar
6. Kane J, Honigfeld G, Singer J, Meltzer H: Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988; 45:789–796Crossref, Medline, Google Scholar
7. Kapur S, Remington G: Serotonin-dopamine interaction and its relevance to schizophrenia. Am J Psychiatry 1996; 153:466–476Link, Google Scholar
8. Ohuoha DC, Hyde TM, Kleinman JE: The role of serotonin in schizophrenia: an overview of the nomenclature, distribution and alterations of serotonin receptors in the central nervous system. Psychopharmacology (Berl) 1993; 112:S5–S15Google Scholar
9. Meltzer HY: The role of serotonin in schizophrenia and the place of serotonin-dopamine antagonist antipsychotics. J Clin Psychopharmacol 1995; 15:2S–3SCrossref, Medline, Google Scholar
10. Carpenter WT Jr: Serotonin-dopamine antagonists and treatment of negative symptoms. J Clin Psychopharmacol 1995; 15:30S–35SCrossref, Medline, Google Scholar
11. Arora RC, Meltzer HY: Serotonin2 receptor binding in blood platelets of schizophrenic patients. Psychiatry Res 1993; 47:111–119Crossref, Medline, Google Scholar
12. Pandey SC, Sharma RP, Janicak PG, Marks RC, Davis JM, Pandey GN: Platelet serotonin-2 receptors in schizophrenia: effects of illness and neuroleptic treatment. Psychiatry Res 1993; 48:57–68Crossref, Medline, Google Scholar
13. Schotte A, Maloteaux JM, Laduron PM: Characterization and regional distribution of serotonin S2 receptors in human brain. Brain Res 1983; 276:231–235Crossref, Medline, Google Scholar
14. Luabeya MK, Maloteaux JM, Laduron PM: Regional and cortical laminar distributions of serotonin S2, benzodiazepine, muscarinic, and dopamine D2 receptors in human brain. J Neurochem 1984; 43:1068–1071Crossref, Medline, Google Scholar
15. Pazos A, Probst A, Palacios JM: Serotonin receptors in the human brain, IV: autoradiographic mapping of serotonin-2 receptors. Neuroscience 1987; 21:123–139Crossref, Medline, Google Scholar
16. Radja F, LaPorte A-M, Daval GV, Verg D, Gozlan H, Hamon M: Autoradiography of serotonin receptor subtypes in the central nervous system. Neurochem Int 1991; 18:1–15Crossref, Medline, Google Scholar
17. Bennett JP, Enna SJ, Bylund DB, Gillin JC, Wyatt RJ, Snyder SH: Neurotransmitter receptors in frontal cortex of schizophrenics. Arch Gen Psychiatry 1979; 36:927–934Crossref, Medline, Google Scholar
18. Whitaker PM, Crow TJ, Ferrier IN: Tritiated LSD binding in frontal cortex in schizophrenia. Arch Gen Psychiatry 1981; 38:278–280Crossref, Medline, Google Scholar
19. Reynolds GP, Rossor MN, Iversen LL: Preliminary studies of human cortical 5-HT2 receptors and their involvement in schizophrenia and neuroleptic drug action. J Neural Transm Suppl 1983; 18:273–277Medline, Google Scholar
20. Mita T, Hanada S, Nishino N, Kuno T, Nakai H, Yamadori T, Mizoi Y, Tanaka C: Decreased serotonin S2 and increased dopamine D2 receptors in chronic schizophrenics. Biol Psychiatry 1986; 21:1407–1414Crossref, Medline, Google Scholar
21. Arora RC, Meltzer HY: Serotonin2 (5-HT2) receptor binding in the frontal cortex of schizophrenic patients. J Neural Transm Gen Sect 1991; 85:19–29Crossref, Medline, Google Scholar
22. Laruelle M, Abi-Dargham A, Casanova MF, Toti R, Weinberger DR, Kleinman JE: Selective abnormalities of prefrontal serotonergic receptors in schizophrenia: a postmortem study. Arch Gen Psychiatry 1993; 50:810–818Crossref, Medline, Google Scholar
23. Joyce JN, Shane A, Lexow N, Winokur A, Casanova MF, Kleinman JE: Serotonin uptake sites and serotonin receptors are altered in the limbic system of schizophrenics. Neuropsychopharmacology 1993; 8:315–336Crossref, Medline, Google Scholar
24. Dean B, Hayes W, Opeskin K, Naylor L, Pavey G, Hill C, Keks N, Copolov DL: Serotonin2 receptors and the serotonin transporter in the schizophrenic brain. Brain Res Behav Brain Res 1996; 73:169–175Crossref, Medline, Google Scholar
25. Dean B, Hayes W: Decreased frontal cortical serotonin2A receptors in schizophrenia. Schizophr Res 1996; 21:133–139Crossref, Medline, Google Scholar
26. Burnet PW, Eastwood SL, Harrison PJ: 5-HT1A and 5-HT2A receptor mRNAs and binding site densities are differentially altered in schizophrenia. Neuropsychopharmacology 1996; 15:442–455Crossref, Medline, Google Scholar
27. Blin J, Pappata S, Kiyosawa M, Crouzel C, Baron JC: [18F]Setoperone: a new high-affinity ligand for positron emission tomography study of the serotonin-2 receptors in baboon brain in vivo. Eur J Pharmacol 1988; 147:73–82Crossref, Medline, Google Scholar
28. Crouzel C, Venet M, Irie T, Sanz G, Boullais C: Labeling of a serotoninergic ligand with [18F]: [18F]setoperone. J Labelled Compounds and Radiopharmaceuticals 1988; 25:403–414Crossref, Google Scholar
29. Maziere B, Crouzel C, Venet M, Stulzaft O, Sanz G, Ottaviani M, Sejourne C, Pascal O, Bisserbe JC: Synthesis, affinity and specificity of 18F-setoperone, a potential ligand for in-vivo imaging of cortical serotonin receptors. Int J Radiation Applications and Instrumentation, part B 1988; 15:463–468Crossref, Medline, Google Scholar
30. Blin J, Sette G, Fiorelli M, Bletry O, Elghozi JL, Crouzel C, Baron JC: A method for the in vivo investigation of the serotonergic 5-HT2 receptors in the human cerebral cortex using positron emission tomography and 18F-labeled setoperone. J Neurochem 1990; 54:1744–1754Crossref, Medline, Google Scholar
31. Seeman P: Receptor Tables: Drug Dissociation Constants for Neuroreceptors and Transporters, vol 2. Toronto, SZ Research, 1993Google Scholar
32. Blin J, Crouzel C: Blood-cerebrospinal fluid and blood-brain barriers imaged by 18F-labeled metabolites of 18F-setoperone studied in humans using positron emission tomography. J Neurochem 1992; 58:2303–2310Crossref, Medline, Google Scholar
33. Kapur S, Jones C, DaSilva J, Wilson A, Houle S: Reliability of a simple non-invasive method for the evaluation of 5-HT2 receptors using [18F]-setoperone PET imaging. Nucl Med Commun 1997; 18:395–399Crossref, Medline, Google Scholar
34. Blin J, Baron JC, Dubois B, Crouzel C, Fiorelli M, Attar-Levy D, Pillon B, Fournier D, Vidailhet M, Agid Y: Loss of brain 5-HT2 receptors in Alzheimer’s disease: in vivo assessment with positron emission tomography and [18F]setoperone. Brain 1993; 116:497–510Crossref, Medline, Google Scholar
35. Kapur S, Zipursky R, Remington G, Jones C, McKay G, Houle S: PET evidence that loxapine is an equipotent blocker of 5-HT2 and D2 receptors: implications for the treatment of schizophrenia. Am J Psychiatry 1997; 154:1525–1529Link, Google Scholar
36. Sadzot B, Lemaire C, Maquet P, Salmon E, Plenevaux A, Degueldre C, Hermanne JP, Guillaume M, Cantineau R, Comar D, Franck G: Serotonin 5HT(2) receptor imaging in the human brain using positron emission tomography and a new radioligand, [F-18]altanserin: results in young normal controls. J Cereb Blood Flow Metab 1995; 15:787–797Crossref, Medline, Google Scholar
37. Crouzel C, Guillaume M, Barre L, Lemaire C, Pike VW: Ligands and tracers for PET studies of the 5-HT system—current status. Int J Radiation Applications and Instrumentation, part B 1992; 19:857–870Crossref, Medline, Google Scholar
38. Wong DF, Lever JR, Hartig PR, Dannals RF, Villemagne V, Hoffman BJ, Wilson AA, Ravert HT, Links JM, Scheffel U, Wagner HN: Localization of serotonin 5-HT2 receptors in living human brain by positron emission tomography using N1-([11C]-methyl)-2-Br-LSD. Synapse 1987; 1:393–398Crossref, Medline, Google Scholar
39. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders (SCID), Clinician Version. Washington, DC, American Psychiatric Press, 1995Google Scholar
40. Opler LA, Kay SR, Lindenmayer JP, Fiszbein A: Structured Clinical Interview for the Positive and Negative Syndrome Scale. Toronto, Multi-Health Systems, 1992Google Scholar
41. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Non-Patient Edition (SCID-NP, Version 1.0). Washington, DC, American Psychiatric Press, 1990Google Scholar
42. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. Stuttgart, Germany, Georg Thieme, 1988Google Scholar
43. Petit-Taboue MC, Landeau B, Osmont A, Tillet I, Barre L, Baron JC: Estimation of neocortical serotonin-2 receptor binding potential by single-dose fluorine-18-setoperone kinetic PET data analysis. J Nucl Med 1996; 37:95–104Medline, Google Scholar
44. Wong DF, Wagner HN Jr, Dannals RF, Links JM, Frost JJ, Ravert HT, Wilson AA, Rosenbaum AE, Gjedde A, Douglass KH: Effects of age on dopamine and serotonin receptors measured by positron tomography in the living human brain. Science 1984; 226:1393–1396Crossref, Medline, Google Scholar
45. Iyo M, Yamasaki T: The detection of age-related decrease of dopamine D1, D2 and serotonin 5-HT2 receptors in living human brain. Prog Neuropsychopharmacol Biol Psychiatry 1993; 17:415–421Crossref, Medline, Google Scholar
46. Wang GJ, Volkow ND, Logan J, Fowler JS, Schlyer D, MacGregor RR, Hitzemann RJ, Gur RC, Wolf AP: Evaluation of age-related changes in serotonin 5-HT2 and dopamine D2 receptor availability in healthy human subjects. Life Sci 1995; 56:PL249–PL253Google Scholar
47. Iqbal N, Asnis GM, Wetzler S, Kahn RS, Kay SR, van Praag HM: The MCPP challenge test in schizophrenia: hormonal and behavioral responses. Biol Psychiatry 1991; 30:770–778Crossref, Medline, Google Scholar
48. Kahn RS, Davidson M: Serotonin receptor responsivity in schizophrenia. Int Clin Psychopharmacol 1993; 8:47–51Crossref, Medline, Google Scholar
49. Krystal JH, Seibyl JP, Price LH, Woods SW, Heninger GR, Aghajanian GK, Charney DS: m-Chlorophenylpiperazine effects in neuroleptic-free schizophrenic patients: evidence implicating serotonergic systems in the positive symptoms of schizophrenia. Arch Gen Psychiatry 1993; 50:624–635Crossref, Medline, Google Scholar
50. Abel KM, O’Keane V, Murray RM: Enhancement of the prolactin response to d-fenfluramine in drug-naive schizophrenic patients. Br J Psychiatry 1996; 168:57–60Crossref, Medline, Google Scholar
51. Abel KM, O’Keane V, Murray RM, Cleare AJ: Serotonergic function and negative and depressive symptomatology in schizophrenia and major depression. Psychoneuroendocrinology 1997; 22:539–548Crossref, Medline, Google Scholar
52. Lindenmayer JP, Adityanjee, Vital-Herne M, Bark N, Grochowski S, Moynihan N: Heterogeneity of serotonergic response in treatment-refractory schizophrenia patients. Biol Psychiatry 1997; 42:6–12Crossref, Medline, Google Scholar
53. Kahn RS, Davidson M, Siever L, Gabriel S, Apter S, Davis KL: Serotonin function and treatment response to clozapine in schizophrenic patients. Am J Psychiatry 1993; 150:1337–1342Link, Google Scholar
54. Curtis VA, Wright P, Reveley A, Kerwin R, Lucey JV: Effect of clozapine on d-fenfluramine-evoked neuroendocrine responses in schizophrenia and its relationship to clinical improvement. Br J Psychiatry 1995; 166:642–646Crossref, Medline, Google Scholar
55. Cohen J: Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ, Lawrence Erlbaum Associates, 1988Google Scholar
56. Andreasen NC, Carson R, Diksic M, Evans A, Farde L, Gjedde A, Hakim A, Lal S, Nair N, Sedvall G, Tune L, Wong DF: Workshop on schizophrenia, PET, and dopamine D2 receptors in the human neostriatum. Schizophr Bull 1988; 14:471–484Crossref, Medline, Google Scholar



