Effect of Acute Metabolic Stress on Pituitary-Adrenal Axis Activation in Patients With Schizophrenia
Abstract
OBJECTIVE: Although several lines of evidence suggest that stress plays a role in the course of schizophrenia, studies that have assessed stress-relevant neurobiological measures have not produced consistent results. The authors examined the effects of acute metabolic stress induced by 2-deoxy-D-glucose (2-DG) on pituitary-adrenal axis activation. METHOD: Thirteen patients with schizophrenia and 11 healthy comparison subjects were administered pharmacological doses of 2-DG (40 mg/kg). The subjects' arterial plasma was then assayed for levels of adrenocorticotropic hormone (ACTH) and cortisol. RESULTS: 2-DG induced significant increases in the measured hormones in both groups, and ACTH elevations were significantly greater in patients with schizophrenia than in comparison subjects. CONCLUSIONS: Patients with schizophrenia have an exaggerated ACTH response to acute metabolic stress exposure. (Am J Psychiatry 1998; 155:979–981)
Several lines of evidence suggest that stress plays a role in the course of schizophrenia. Illness onset (1) and relapse (2) are associated with environmental stress. Stress-reducing behavioral (3) and pharmacological treatments (4) may improve the symptoms of schizophrenia. Moreover, hypercortisolism, e.g., elevated plasma cortisol (5) or cortisol nonsuppression after dexamethasone administration (5, 6), has been reported in schizophrenia and is potentially related to stress and arousal (5, 7, 8).
Relatively few studies have exposed patients with schizophrenia to laboratory-induced stress to determine if there are neurobiological correlates to stress response, and these studies have yielded inconsistent results (9, 10). One method of acute stress induction is administration of pharmacological doses of 2-deoxy-D-glucose (2-DG). 2-DG is a glucose analog that blocks glycolysis by inhibition of glucose-6-phosphate dehydrogenase, thus preventing neuronal glucose utilization and resulting in a clinical state similar to hypoglycemia (11). Although metabolic stress may be distinct from the environmental stress implicated in the schizophrenia literature, it offers the advantage of activation of stress-related neurochemical and neuroendocrine systems, including the pituitary-adrenal axis (12), and may provide unique information pertaining to regulatory mechanisms of hormonal synthesis, release, and clearance.
The present study investigated the effects of 2-DG administration on arterial plasma levels of the pituitary-adrenal axis hormones ACTH and cortisol in patients with schizophrenia and healthy comparison subjects. The advantage of arterial sampling is that it is less affected by local peripheral metabolism, as suggested by preclinical studies demonstrating that muscle and skin tissues remove more than 70% of intravenously injected radioactive-labeled ACTH within 1 minute (13). Arterial plasma is used in endocrine research of the sympathoneural system; therefore, it may have heuristic value in the investigation of the anatomically and functionally related hypothalamic-pituitary-adrenal axis (14).
METHOD
Thirteen outpatients who met DSM-IV criteria for schizophrenia (mean age at appearance of DSM-IV criterion A symptoms of schizophrenia=22.5 years, SD=3.5; mean duration of the illness=15.7 years, SD=9.3) and 11 healthy comparison subjects participated in this study. The patients were diagnosed by two research psychiatrists using a best-estimate format that uses all available sources of information, including clinical history, interview, and the Structured Clinical Interview for DSM-III-R. The patients were tested while receiving stable doses of typical antipsychotic drugs for a minimum of 2 weeks (except for one patient, who had received the drug for 8 days). Drugs and doses (chlorpromazine equivalent mean=748.54 mg/day, SD=501.31, range=333–2000) were varied to achieve a stable clinical condition. Comparison subjects were recruited through the normal volunteer program of the National Institutes of Health (NIH) and had no psychiatric history. All individuals were in good physical health and gave written informed consent to a protocol approved by the NIH Institutional Review Board.
On the morning of the procedure, subjects were admitted to the 4 East Unit of NIH, having fasted and refrained from alcohol, tobacco, caffeine, and physical activity for at least 10 hours. An arterial line was placed under local anesthesia with 1% lidocaine. After a 90-minute rest period, 2-DG (40 mg/kg, maximal dose 4000 mg) was administered as an intravenous bolus. Blood samples were obtained at 30 minutes before (–30 minutes), immediately prior (0 minutes), and 20, 40, and 60 minutes following 2-DG administration. The assays for ACTH and cortisol were performed as described elsewhere (15).
The data were analyzed by using the statistical package SuperANOVA (16). Baseline hormonal levels were determined from the mean of hormonal levels at the –30-minute and 0-minute time points. Analyses were conducted by using two-way analysis of variance (ANOVA) with repeated measures design. Diagnosis was a grouping factor, and time was the within-subject factor. When group-by-time interactions were significant, post hoc Newman-Keuls t tests were performed. All ANOVAs were interpreted with the Greenhouse-Geisser conservative F test to account for sphericity. All analyses were two-tailed. A p value less than 0.05 defined statistical significance. Group data were summarized as means and standard deviations.
RESULTS
There were no significant differences between patients with schizophrenia and comparison subjects in age (mean=37.9 years, SD=8.7, versus mean=32.4 years, SD=6.5) (t=1.7, df=22, p=0.10), gender (10 men and three women versus nine men and two women) (χ2=0.09, df=1, p=0.77), and weight (mean=81.5 kg, SD=13.5, versus mean=78.2 kg, SD=18.4) (t=0.5, df=22, p=0.61).
Patients with schizophrenia and comparison subjects both demonstrated robust increases in plasma ACTH levels (mean change from baseline: 797% versus 386%). Patients demonstrated significantly higher 2-DG-induced ACTH levels than comparison subjects (figure 1). Post hoc Newman-Keuls t tests revealed no differences between groups in ACTH levels at baseline (mean=28.68 pg/ml, SD=17.37, versus mean=40.23 pg/ml, SD=15.91) (p=0.77) but did show significantly higher ACTH levels for patients at 20 minutes (p<<0.01), 40 minutes (p<0.01), and 60 minutes (p<0.01) after the ad~ministration of 2-DG.
Analyses of cortisol data revealed similar baseline levels in patients and comparison subjects (mean=13.99 µg/dl, SD=8.19, versus mean=13.52 µg/dl, SD=5.17). 2-DG produced a significant time effect but no group effect or group-by-time interaction (figure 1). All subjects demonstrated usual behavioral and clinical responses to 2-DG administration (12).
DISCUSSION
The major finding of this study is that patients with schizophrenia had significantly greater 2-DG-induced plasma ACTH levels but not cortisol levels than healthy comparison subjects. To our knowledge, this is the first report of exaggerated ACTH response to pharmaco~logical challenge in patients with schizophrenia.
Our findings are consistent with other reports of pituitary-adrenal axis abnormalities in schizophrenia, such as basal hypercortisolemia (5) and cortisol nonsuppression after dexamethasone (8). Our results contrast with the finding of a clinical study using 2-DG as a metabolic stressor. Breier (12) found that 2-DG did not induce significantly different ACTH responses in patients with schizophrenia and comparison subjects. These contrasting data might be accounted for by differences in study design, patient selection, method of 2-DG administration (bolus versus infusion), and sampling of arterial rather than venous blood.
The unexpected lack of significant group differences in 2-DG-induced cortisol elevations in the face of ACTH response may have been due to a “ceiling effect.” Cortisol elevations exceeded the threshold for maximal adrenal response (17), and cortisol levels reached the 95th percentile value achieved in healthy comparison subjects on a standard ACTH stimulation test (18). This hypothesis, however, requires further investigation given the small number of subjects in our study and evidence that cortisol levels did appear to be higher, although not statistically significantly higher, in the patients. In addition, this study assessed only acute stress response, and a longer study period may have yielded different results because cortisol levels were still rising at the end of the experiment.
The following caveats should be considered in interpreting our results. Because of the magnitude of the 2-DG response and the lack of group differences in hormonal levels at baseline, it is unlikely that basal endocrine status contributed to the 2-DG-induced endocrine effects. It is also unlikely that antipsychotic drugs affected the ACTH response to 2-DG. Clinical and preclinical studies have shown that chronic antipsychotic drug treatment is not associated with ACTH elevations (5, 19) but might have a dampening effect on pituitary-adrenal axis activity (5). Nonetheless, a study of antipsychotic-drug-free patients may be warranted.
In conclusion, 2-DG-induced metabolic stress elicited a heightened ACTH response in patients with schizophrenia. These data suggest that elucidation of the mechanisms underlying the metabolic stress response may provide information that would help us understand the pathophysiology and treatment of the illness.
Received July 15, 1997; revisions received Dec. 24, 1997, and Feb. 6, 1998; accepted Feb. 16, 1998. From the Experimental Therapeutics Branch, NIMH, National Institutes of Health, Bethesda, Md. Address reprint requests to Dr. Elman, Massachusetts General Hospital, Harvard Medical School, West End House, 16 Blossom St., Boston, MA 02114; [email protected] (e-mail). The authors thank Lisa Picken, Kingsley Turner, Jerry Rotter, and Allan Clifton for their contribution to this study and Marilyn Duncan for editorial assistance.
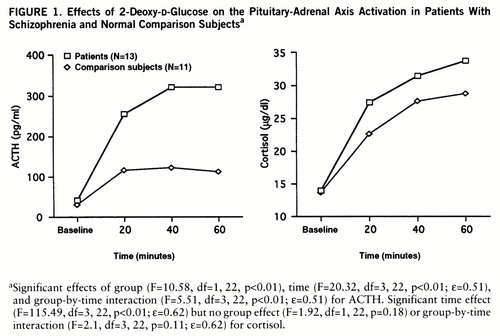
FIGURE 1. Effects of 2-Deoxy-d-Glucose on the Pituitary-Adrenal Axis Activation in Patients With Schizophrenia and Normal Comparison Subjectsa
aSignificant effects of group (F=10.58, df=1,22, p<0.01), time (F=20.32, df=3,22, p<0.01; ε=0.51), and group-by-time interaction (F=5.51, df=3,22, p<0.01; ε=0.51) for ACTH. Significant time effect (F=115.49, df=3,22, p<0.01; ε=0.62) but no group effect (F=1.92, df=1,22, p=0.18) or group-by-time interaction (F=2.1, df=3,22, p=0.11; ε=0.62) for cortisol.
1 Gruen R, Baron M: Stressful life events and schizophrenia: relation to illness onset and family history. Neuropsychobiology 1984; 12:206–208Crossref, Medline, Google Scholar
2 Hultman CM, Wieselgren IM, Ohman A: Relationships between social support, social coping and life events in the relapse of schizophrenic patients. Scand J Psychol 1997; 38:3–13Crossref, Medline, Google Scholar
3 Starkey D, Deleone H, Flannery RBJ: Stress management for psychiatric patients in a state hospital setting. Am J Orthopsychiatry 1995; 65:446–450Crossref, Medline, Google Scholar
4 Wolkowitz OM, Pickar D: Benzodiazepines in the treatment of schizophrenia: a review and reappraisal. Am J Psychiatry 1991; 148:714–726Link, Google Scholar
5 Lammers CH, Garcia-Borreguero D, Schmider J, Gotthardt U, Dettling M, Holsboer F, Heuser IJ: Combined dexamethasone/corticotropin-releasing hormone test in patients with schizophrenia and in normal controls, II. Biol Psychiatry 1995; 38:803–807Crossref, Medline, Google Scholar
6 Asnis GM, Eisenberg J, Lemus CZ, Halbreich U: Dexamethasone suppression test in schizophrenia: a study and review. Neuropsychobiology 1986; 15:109–113Crossref, Medline, Google Scholar
7 Yeragani VK: The incidence of abnormal dexamethasone suppression in schizophrenia: a review and a meta-analytic comparison with the incidence in normal controls. Can J Psychiatry 1990; 35:128–132Crossref, Medline, Google Scholar
8 Tandon R, Mazzara C, DeQuardo J, Craig KA, Meador-Woodruff JH, Goldman R, Greden JF: Dexamethasone suppression test in schizophrenia: relationship to symptomatology, ventricular enlargement, and outcome. Biol Psychiatry 1991; 29:953–964Crossref, Medline, Google Scholar
9 Albus M, Engel RR, Muller F, Zander KJ, Ackenheil M: Experimental stress situations and the state of autonomic arousal in schizophrenic and depressive patients. Int Pharmacopsychiatry 1982; 17:129–135Crossref, Medline, Google Scholar
10 Breier A, Wolkowitz OM, Doran AR, Bellar S, Pickar D: Neurobiological effects of lumbar puncture stress in psychiatric patients and healthy volunteers. Psychiatry Res 1988; 25:187–194Crossref, Medline, Google Scholar
11 Horton RW, Meldrum BS, Bachelard HS: Enzymic and cerebral metabolic effects of 2-deoxy-D-glucose. J Neurochem 1973; 21:507–520Crossref, Medline, Google Scholar
12 Breier A: Experimental approaches to human stress research: assessment of neurobiological mechanisms of stress in volunteers and psychiatric patients. Biol Psychiatry 1989; 26:438–462Crossref, Medline, Google Scholar
13 Ambler L, Bennett HP, Hudson AM, McMartin C: Fate of human corticotrophin immediately after intravenous administration to the rat. J Endocrinol 1982; 93:287–292Crossref, Medline, Google Scholar
14 Goldstein DS: Stress, Catecholamines, and Cardiovascular Disease. New York, Oxford University Press, 1995Google Scholar
15 Elman I, Breier A: Effects of metabolic stress on plasma progesterone and testosterone in human subjects: relationship to pituitary-adrenocortical axis. Life Sci 1997; 61:1705–1712Crossref, Medline, Google Scholar
16 SuperANOVA. Berkeley, Calif, Abacus Concepts, 1989Google Scholar
17 Graybeal ML, Fang VS: Physiological dosing of exogenous ACTH. Acta Endocrinol 1985; 108:401–406Crossref, Medline, Google Scholar
18 Crowley S, Hindmarsh PC, Honour JW, Brook CG: Reproducibility of the cortisol response to stimulation with a low dose of ACTH(1-24): the effect of basal cortisol levels and comparison of low-dose with high-dose secretory dynamics. J Endocrinol 1993; 136:167–172Crossref, Medline, Google Scholar
19 Meador-Woodruff JH, Greden JF: Effects of psychotropic medications on hypothalamic-pituitary-adrenal regulation. Neurol Clin 1988; 6:225–234Crossref, Medline, Google Scholar



