Effects of Antidepressant Treatment on Neuroactive Steroids in Major Depression
Abstract
OBJECTIVE: There is evidence from animal studies that fluoxetine may enhance the concentrations of neuroactive steroids. Therefore, the authors investigated whether clinically effective treatment with antidepressants may alter the concentrations of neuroactive steroids in patients suffering from a major depressive episode. METHOD: In the first study, eight drug-naive outpatients with major depression were studied during treatment with fluoxetine. In a complementary study, 11 inpatients with major depression were studied during a severe depressive episode and after recovery following treatment with different antidepressants. Plasma samples were quantified for neuroactive steroids by means of a highly sensitive and specific combined gas chromatography/mass spectrometry analysis. RESULTS: During depression, there was a significant decrease in 3α, 5α-tetrahydroprogesterone (3α, 5α-THP) and 3α, 5β-THP concentrations, both of which are positive modulators of the γ-aminobutyric acidA receptor, and a concomitant increase in 3β, 5α-THP levels. This dysequilibrium of neuroactive steroids could be corrected by treatment with different antidepressants. CONCLUSIONS: These results provide the first clinical evidence of a possible role of neuroactive steroids in successful antidepressant therapy. (Am J Psychiatry 1998; 155:910–913)
The efficacy of therapy with antidepressants is usually attributed to their inhibitory action on neurotransmitter transporters or the blockade of monoamine oxidase (1). However, it has been suggested that antidepressants may also work by decreasing the hyperactivity of the hypothalamic-pituitary-adrenocortical (HPA) system (2). Recent evidence from animal studies suggests that the selective serotonin reuptake inhibitor (SSRI) fluoxetine increases the concentrations of certain neuroactive steroids that interact with the γ-amino~butyric acid (GABA)A/benzodiazepine receptor complex (3). These steroids are potent positive allosteric modulators of the GABAA receptor (4–6) and may also regulate gene expression by means of the progesterone receptor after intracellular oxidation (6, 7). Moreover, GABA-ergic neuroactive steroids may decrease the activity of the HPA system (6, 8). To challenge the question of whether treatment with antidepressants may act through changes in the composition of different neuroactive steroids, we measured their concentrations in plasma samples of drug-naive depressed outpatients before and during treatment with fluoxetine. In a second group, we studied inpatients during a severe depressive episode and after clinical recovery following effective treatment with various antidepressants other than SSRIs.
METHOD
In the first study, eight male depressed outpatients with the first episode of a unipolar major depressive disorder according to DSM-IV (mean age=34.6 years, SD=16.5) were examined. These patients had no previous history of psychiatric disorders and had never been treated with psychopharmacological drugs or psychotherapy. Eight healthy men (mean age=34.7 years, SD=15.8) served as control subjects. After blood sampling at day 0, each patient was treated, in an outpatient setting, with 20 mg/day of fluoxetine, administered in the early morning until day 50. Severity of depression was assessed by the 17-item Hamilton Depression Rating Scale. The mean Hamilton depression scale scores were 20.7 (SD=4.4) at day 0 and 9.4 (SD=6.0) at day 50. Plasma samples were obtained at 9:00 a.m. on day 0 and on every 10 days of treatment until day 50 and were quantified for progesterone, 5α-pregnan-3α-ol-20-one (3α, 5α-THP), 5β-pregnan-3α-ol-20-one (3α, 5β-THP), and 5α-pregnan-3β-ol-20-one (3β, 5α-THP).
In the second study, 11 severely depressed inpatients with a major depressive disorder according to DSM-IV (three men and eight women, mean age=53.0 years, SD=10.8) were examined. At baseline examination, these patients had been medication free for at least 4 days. Four of the women were tested during the midluteal phase of the cycle; the other women were postmenopausal. Severity of depression was assessed by the 21-item Hamilton depression scale. The mean Hamilton depression scale scores of the depressed patients were 28.1 (SD=4.3) at baseline and 5.3 (SD=4.1) after recovery. In this clinical setting the treating clinician was free to decide the type of psychopharmacotherapy used until clinical recovery. Remission was achieved in five patients with amitriptyline (75–200 mg/day), in three patients with clomipramine (75–100 mg/day), and in one each with nortriptyline (480 mg/day), viloxazine (400 mg/day), and lithium (600 mg/day). The mean duration of treatment was 55.0 days (SD=10.4). Plasma samples were obtained at 4:00 p.m., before and after treatment, and were quantified for pregnenolone, progesterone, dehydroepiandrosterone (DHEA), 5α-pregnan-3, 20-dione (5α-DHP), 3α, 5α-THP, 3α, 5β-THP, and 3β, 5α-THP.
The subjects of both studies had undergone a thorough medical examination to rule out other illness, drug intake, and lifestyles that could interfere with the study. The protocols were approved by the local ethics committee for human experiments, and written informed consent was obtained from each participant before the investigation.
Steroid determinations were performed by using a highly sensitive and specific combined gas chromatography/mass spectrometry analysis (9). Briefly, after extraction with 3×2 ml of ethyl acetate and separation by thin layer chromatography (i.e., carbon tetrachloride/methanol [99:1, vol/vol], cyclohexane/ethyl acetate [3:2, vol/vol]), the eluate containing 3α, 5α-THP, 3α, 5β-THP, 3β, 5α-THP, progesterone, pregnenolone, and DHEA was lyophilized and derivatized with heptafluorobutyric acid anhydride. 5α-DHP was derivatized with methoxamine hydrochloride (2%) in pyridine. Heptafluorobutyric acid anhydride and methoxamine derivatives were analyzed by gas chromatography/mass spectrometry by using a Hewlett-Packard 5971 mass selective detector coupled to a 5890A gas chromatograph equipped with a capillary column (Hewlett-Packard-5 mass spectrometer; length, 30 m; internal diameter, 0.25 mm; film thickness, 0.25 µm). The steroids were assayed in the electron impact mode, and the ions at m/z (ratio of fragment mass [m] to atomic number [z]) 510, 496, 298, 270, and 343 were selectively monitored.
Differences in the steroid concentrations between depressed outpatients and control subjects (group effect) at day 0, as well as at day 50 after treatment with fluoxetine, were tested for significance with the Wilks's multivariate test by applying in both cases a one-factorial multivariate analysis of variance (MANOVA) with group as a between-subjects factor. By a significant group effect, the identification of steroids that contributed significantly to this effect was carried out by subsequent univariate F tests. Possible differences in steroid concentrations during fluoxetine treatment within the outpatient group were tested for significance by using for each steroid a one-factorial repeated measures analysis of variance (ANOVA) with time as within-subjects factor with six levels. Tests with contrasts were subsequently performed for the steroids that revealed a significant time effect in order to locate the time points with significant differences in their mean values in comparison to day 0. Mean steroid concentrations of depressed inpatients during depression and after clinical remission were tested for significance by another one-factorial MANOVA with repeated measures design. Treatment was then the within-subjects factor with two levels. When a treatment effect was found, univariate F tests followed to identify those steroids that contributed significantly to the treatment differences. To increase homogeneity and to approach normality of the data, the variables used in the analysis were transformed by log transformation (x*=ln[x+1], where x*=transformed values and ln=the natural logarithm) before analysis. An alpha level of 0.05 was accepted as a nominal level of significance. To keep the type I error at 0.05 or less, all a posteriori tests (univariate F tests and tests with contrasts) were performed at a reduced level of significance (alpha adjusted according to the Bonferroni procedure). Correlation coefficients for steroid concentrations and Hamilton depression scale scores were obtained by Spearman's rank correlation.
RESULTS
In the comparison of depressed outpatients and control subjects on day 0, ANOVA revealed a significant group effect (Wilks's multivariate test; effect of group: F=8.15, df=4,11, p=0.003), which was attributable to significant differences between depressed patients and control subjects in the concentrations of 3α, 5α-THP, 3α, 5β-THP, and 3β, 5α-THP (univariate F tests, df=1,14: minimum of the corresponding F values=5.39, p<0.05), but not in those of progesterone. No significant group effect could be detected by MANOVA between outpatients at day 50 following fluoxetine treatment and control subjects (F=0.94, df=4,11, p>0.05).
In the depressed outpatients treated with fluoxetine, ANOVA revealed a significant time effect for 3α, 5α-THP, 3α, 5β-THP, and 3β, 5α-THP (Wilks's multivariate test; effect of time: minimum of the corresponding F values=11.62, df=5,3, p<0.05). The levels of 3α, 5α-THP and 3α, 5β-THP increased continuously, while there was a concomitant decrease in 3β, 5α-THP concentrations (figure 1). The changes between day 0 and subsequent measurements of these steroids reached statistical significance from day 30 (tests with contrasts: 3α, 5α-THP: F=35.91, df=1,7, p<0.05; 3α, 5β-THP: F=27.61, df=1,7, p<0.05; 3β, 5α-THP: F=16.78, df=1,7, p<0.05). There was no significant correlation between the pre- and posttreatment difference in the Hamilton depression scale score and the pre- and posttreatment difference in the concentrations of either steroid.
Among the inpatients the comparison of posttreatment steroid concentrations with the mean concentrations during depression revealed a significant treatment effect (Wilks's multivariate test; effect of treatment: F=7.8, df=7,4, p=0.03, MANOVA). The treatment effect (table 1) was attributable both to the significant increase in the 3α, 5α-THP and 3α, 5β-THP levels after successful antidepressant therapy and to the significant decrease in the 3β, 5α-THP level after clinical remission. Pregnenolone, DHEA, progesterone, and 5α-DHP concentrations did not change significantly after clinical recovery achieved by treatment with antidepressants. There was a significant negative correlation between the duration of the drug-free interval before the study and baseline 3α, 5α-THP levels (r=–0.68, N=11, p<0.05). However, no correlation could be established between the rate of improvement in the Hamilton depression scale score and the changes in steroid concentrations following treatment with antidepressants.
DISCUSSION
During a major depressive episode, we found a decrease in the plasma concentrations of 3α, 5α-THP and 3α, 5β-THP, which are positive allosteric modulators of the GABAA receptor (4–6), and a concomitant increase in 3β, 5α-THP, which may act as a functional antagonist for GABA-agonistic steroids (10), in view of unchanged progesterone concentrations. These findings suggest that progesterone metabolism is dysregulated during depression, possibly resulting in a decreased GABA-ergic neurotransmission. The alterations in the composition of neuroactive steroids could be corrected by treatment with various antidepressants. No significant changes after clinical remission were observed with regard to pregnenolone, DHEA, progesterone, or 5α-DHP concentrations. Although our findings must be considered as preliminary because of the small group sizes, they suggest that an increase in 3α, 5α-THP and 3α, 5β-THP, together with a decrease in 3β, 5α-THP, contributes to the successful treatment of major depressive episodes with antidepressants. Although an increase in 3α, 5α-THP in the rat brain has been postulated as being specific for SSRI administration (3), our results indicate that changes in plasma concentrations of neuroactive steroids during antidepressant treatment are not restricted to serotonin-reuptake inhibition but are a common feature of treatment with antidepressants.
The molecular mechanisms underlying the treatment-induced changes in the concentrations of neuroactive steroids are still unclear at present. It appears that antidepressant treatment may shift the activity of the 3α-hydroxysteroid oxidoreductase in the reductive direction and thus may enhance the conversion of 5α-DHP into GABAA receptor-modulating 3α, 5α-THP and 3α, 5β-THP (7, 11), a finding that is also supported by animal studies (3). Since it has been suggested that an increased release of corticotropin-releasing hormone (CRH) plays a role in the pathophysiology of depression (12, 13) and 3α, 5α-THP may decrease hypothalamic CRH concentrations (8), an attenuation of the activity of the HPA system might mediate, in part, putative antidepressant effects of such neuroactive steroids. The enhancement of the neuronal response to GABA induced by neuroactive steroids may also contribute to their clinical effects. Studies evaluating the effects of progesterone as a precursor of 3α, 5α-THP and 3α, 5β-THP on sleep (14, 15) revealed effects similar to those of benzodiazepines and therefore support this hypothesis.
A previous report suggested decreased levels of preg~nenolone in the CSF of depressed patients (16). In that study GABA-agonistic neuroactive steroids were not measured, but it has recently been postulated that a decreased availability of such steroids is related to late luteal dysphoric symptoms (17). Moreover, a differential modulation of brain GABAA receptors during the estrus cycle has recently been demonstrated (18). Although the concentrations of neuroactive steroids in the brain are somewhat higher than plasma levels, possibly because of brain-derived synthesis and tissue accumulation, plasma levels are likely to reflect brain levels because these steroids can easily cross the blood brain barrier (5, 6, 14).
The present studies leave open whether the observed alterations in the composition of neuroactive steroids are due to the pharmacological actions of antidepressants or to clinical remission. However, several observations argue for a pharmacological effect: the steroid changes were not related to treatment outcome and were more pronounced in the drug-naive outpatient group, although these patients were less severely depressed; the negative correlation between the length of the drug-free interval and 3α, 5α-THP levels in the inpatient group; and the lack of correlations between the rate of clinical improvement and the steroid changes in both studies. Thus, it would be interesting to evaluate the composition of neuroactive steroids in nonpharmacological treatments of depression such as ECT and cognitive behavioral therapy.
Whether neuroactive steroids have antidepressant properties per se has, to our knowledge, never been studied. However, our findings give the first clinical evidence for a putative role of neuroactive steroids in the treatment of major depression with antidepressants.
 |
Received March 13, 1997; revisions received Aug. 25, 1997, and Jan. 20, 1998; accepted Feb. 26, 1998. From the Department of Experimental Medicine and Department of Psychiatry, Tor Vergata University of Rome, Rome, Italy; and the Max Planck Institute of Psychiatry, Munich. Address reprint requests to Dr. Rupprecht, Department of Psychiatry, Ludwig Maximilian University, Nussbaumstr. 7, 80336 Munich, Germany. Supported by the Gerhard Hess Program of the Deutsche Forschungsgemeinschaft (Dr. Rupprecht). The authors thank Alexander Yassouridis, Ph.D., for statistical advice.
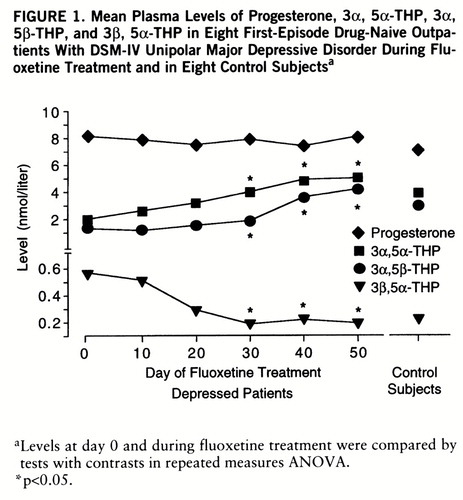
FIGURE 1. Mean Plasma Levels of Progesterone, 3α, 5α-THP, 3α, 5β-THP, and 3β, 5α-THP in Eight First-Episode Drug-Naive Outpatients With DSM-IV Unipolar Major Depressive Disorder During Fluoxetine Treatment and in Eight Control Subjectsa
aLevels at day 0 and during fluoxetine treatment were compared by tests with contrasts in repeated measures ANOVA.
*p<0.05.
1 Burke MJ, Preskorn SH: Short-term treatment of mood disorders with standard antidepressants, in Psychopharmacology: The Fourth Generation of Progress. Edited by Bloom F, Kupfer D. New York, Raven Press, 1995, pp 1053–1065Google Scholar
2 Holsboer F, Barden N: Antidepressants and hypothalamic-pituitary-adrenocortical regulation. Endocr Rev 1996; 17:187–205Crossref, Medline, Google Scholar
3 Uzunov DP, Cooper TB, Costa E, Guidotti A: Fluoxetine-elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. Proc Natl Acad Sci USA 1996; 93:12599–12604Crossref, Medline, Google Scholar
4 Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM: Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 1986; 232:1004–1007Crossref, Medline, Google Scholar
5 Paul SM, Purdy RH: Neuroactive steroids. FASEB J 1992; 6:2311–2322Crossref, Medline, Google Scholar
6 Rupprecht R: The neuropsychopharmacological potential of neuroactive steroids. J Psychiatr Res 1997; 31:297–314Crossref, Medline, Google Scholar
7 Rupprecht R, Reul JMHM, Trapp T, van Steensel B, Wetzel C, Damm K, ZieglgÄnsberger W, Holsboer F: Progesterone receptor mediated effects of neuroactive steroids. Neuron 1993; 11:523–530Crossref, Medline, Google Scholar
8 Patchev VK, Shoaib M, Holsboer F, Almeida OFX: The neuro~steroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience 1994; 62:265–271Crossref, Medline, Google Scholar
9 Romeo E, Cheney DL, Zivkovic I, Costa E, Guidotti A: Mitochondrial diazepam-binding inhibitor receptor complex agonists antagonize dizocilpine amnesia: putative role for allopregnano~lone. J Pharmacol Exp Ther 1994; 270:89–96Medline, Google Scholar
10 Prince RJ, Simmonds MA: 5β-Pregnan-3β-ol-20-one, a specific antagonist at the neurosteroid site of the GABAA receptor complex. Neurosci Lett 1992; 135:273–275Crossref, Medline, Google Scholar
11 Krause JE, Karavolas HJ: Pituitary 5α-dihydroprogesterone 3α-hydroxysteroid oxidoreductases. J Biol Chem 1980; 255:11807–11814Medline, Google Scholar
12 Holsboer F, Spengler D, Heuser IJ: The role of corticotropin-releasing hormone in the pathogenesis of Cushing's disease, anorexia nervosa, alcoholism, affective disorders and dementia. Prog Brain Res 1992; 93:385–417Crossref, Medline, Google Scholar
13 Nemeroff CB, Widerlöv E, Bisette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W: Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science 1984; 226:1342–1343Crossref, Medline, Google Scholar
14 Lancel M, Faulhaber J, Holsboer F, Rupprecht R: Progesterone induces changes in sleep EEG comparable to those of agonistic GABAA receptor modulators. Am J Physiol 1996; 271:E763–E772Google Scholar
15 Friess E, Tagaya H, Trachsel L, Holsboer F, Rupprecht R: Progesterone-induced changes in sleep in male subjects. Am J Phys~iol 1997; 272:E885–E891Google Scholar
16 George MS, Guidotti A, Rubinow D, Pan B, Mikalauskas K, Post RM: CSF neuroactive steroids in affective disorders: preg~nenolone, progesterone, and DBI. Biol Psychiatry 1994; 35:775–780Crossref, Medline, Google Scholar
17 Wang M, Seippel L, Purdy RH, BÄckström T: Relationship between symptom severity and steroid variation in women with premenstrual syndrome: study on serum pregnenolone, pregnenolone sulfate, 5α-pregnane-3, 20-dione and 3α-hydroxy-5α-pregnan-20-one. J Clin Endocrinol Metab 1996; 81:1076–1082Medline, Google Scholar
18 McCauley LD, Gee KW: Influence of the estrus cycle on the discrimination of apparent neuroactive steroid site subtypes on the γ-aminobutyric acidA receptor complex in the rat. J Pharmacol Exp Ther 1995; 275:1412–1417Medline, Google Scholar



