Association Between Decline in Brain Dopamine Activity With Age and Cognitive and Motor Impairment in Healthy Individuals
Abstract
OBJECTIVE: Although it is documented that brain dopamine activity declines with age, the functional significance of this is not known. This study assessed the relation between measures of brain dopamine activity and indexes of motor and cognitive function in healthy individuals. METHOD: Thirty healthy volunteers aged 24–86 years were studied with positron emission tomography and [11C]raclopride to assess dopamine D2 receptors. All subjects underwent a neuropsychological test battery that included tasks found to be sensitive to dopamine alterations in patients with neurodegenerative disease and control tasks. RESULTS: Transfer of [11C]~raclopride from plasma to brain in the striatum and cerebellum was not affected by age. In contrast, D2 receptor availability in the caudate and putamen declined with age. Correlations between D2 receptors and neuropsychological test performance were strongest for the motor task (Finger Tapping Test) and were also significant for most tasks involving frontal brain regions, including measures of abstraction and mental flexibility (Wisconsin Card Sorting Test) and attention and response inhibition (Stroop Color-Word Test, interference score). These relationships remained significant after control for age effects. CONCLUSIONS: Age-related decreases in brain dopamine activity are associated with a decline in motor function and may also contribute to impaired performance on tasks that involve frontal brain regions. Interventions that enhance dopamine activity may improve performance and quality of life for the elderly. The fact that correlations remained significant after age effects were partialed out suggests that dopamine activity may influence motor and cognitive performance irrespective of age.
The progressive increase in the elderly population places a sense of urgency on understanding the neurochemical changes accompanying normal aging and the relationship of these changes to the clinical manifestations that limit performance in later life. Of the age-related neurochemical changes in the brain, those involving the dopamine system appear to be of paramount importance (1, 2). However, despite a large number of studies documenting decreased dopamine activity with aging, very little is known about its functional significance. Part of the difficulty has been that until recently, measurements were done in postmortem brains for which neurocognitive assessment was not available (3). With the development of imaging techniques such as positron emission tomography (PET), it is now possible to measure dopamine-specific parameters in living human subjects and to evaluate in parallel their cognitive and motor performance (4).
Imaging studies of the dopamine system have documented a substantial decline in dopamine D2 receptors (reviewed in reference 5), dopamine D1 receptors (6), and dopamine transporters (reviewed in reference 7), while studies of dopamine metabolism have been less consistent, showing decreases as well as no changes with aging (reviewed in reference 8). However, the functional significance of these changes in the normal human brain has not been investigated. The purpose of this study was to examine the relation between changes in dopamine D2 receptors and cognitive and motor function in healthy individuals.
METHOD
Thirty healthy right-handed volunteers (24 men and six women) were recruited by the Clinical Research Center at Brookhaven National Laboratory. Their mean age was 49 years (SD=18, range=24–86). Twenty were Caucasian, five were African American, three were Asian, and two were Hispanic. Their mean education level was 15 years (SD=3), and their mean verbal IQ was 118 (SD=15). They underwent a complete medical, psychiatric, and neurological examination to ensure the absence of disease. In addition, the inclusion criteria required weight within 20% of the Metropolitan Life Insurance norms and a score of less than 14 on the Hamilton Depression Rating Scale (9). The exclusion criteria included a past or present psychiatric or neurologic disorder, alcohol or substance abuse (except for caffeine and nicotine), medical conditions that could alter cerebral functioning (i.e., immunologic, metabolic, cardiovascular disorders), and a history of head trauma with loss of consciousness. Persons with abnormalities on magnetic resonance imaging (MRI) scans (apart from age-appropriate cortical atrophy) were excluded. None of the subjects was taking medication at the time of the study, and pre-scan urine tests were done to ensure the absence of psychoactive drug use. Informed consent was obtained following guidelines set by the Human Studies Review Committee at Brookhaven National Laboratory.
All subjects completed a neuropsychological test battery that included tasks thought to require substantial input from dopamine pathways (“dopamine-sensitive” tasks) as well as tests thought to be insensitive to dopamine (“control” tasks) (table 1). Tests related to dopamine activity were selected from measures of motor and executive functions (concept formation, abstraction, attention) that have been found to be abnormal in patients with neurodegenerative disease states of the dopamine system (22–24). All tests were administered by an experienced clinical neuropsychologist, and the scoring was checked by an independent examiner.
Scans were done with a CTI 931 tomograph (6×6×6.5 mm full width at half maximum, 15 slices) and [11C]raclopride (25). Details of procedures for positioning, arterial and venous catheterization, quantification and metabolite analyses, and transmission and emission scans have been published (25). Briefly, sequential dynamic scans were started immediately after intravenous injection of 4–10 mCi of [11C]raclopride (specific activity >0.25 Ci/µmol at the time of injection) for a total of 60 minutes.
Regions of interest were drawn directly on the emission scans, since for these dopamine tracers this strategy provides measures equivalent to those obtained when the MRI is used as the template (26). Regions of interest for the caudate, putamen, and cerebellum were obtained with the use of procedures previously described (25). The time-activity curves for [11C]raclopride in the caudate, putamen, and cerebellum and the time-activity curves for unchanged tracer in plasma were used to calculate distribution volumes by means of a graphical analysis technique for reversible systems (Logan plots) (27). The parameter Bmax/Kd—the ratio of the distribution volume in the striatum to that in the cerebellum minus 1—was used as the model of dopamine D2 receptor availability. This parameter is insensitive to changes in cerebral blood flow (28).
Pearson product-moment correlation analyses were performed between age and k1 (the transfer constant of radiotracer from blood to brain), between age and dopamine D2 receptor availability, and between dopamine D2 receptors and neuropsychological test results. To evaluate the influence of education, we also evaluated the correlations for years of education. Because neuropsychological measures show age-related decline (29), we also performed partial correlations to control for age effects on those tests for which the correlations were significant (p<0.01). Partial correlations were considered significant at p<0.05 (two-tailed).
In addition, we used a factor analysis (varimax rotation) to assess the categorization of the neuropsychological tests, and using the scores from the 20 neuropsychological tests, we did a step-down regression analysis to determine the combination of variables that would best predict the dopamine D2 measures. These two multivariate tests were computed for those subjects who completed all of the tests (N=21).
RESULTS
The correlation analysis between age and k1 (the transfer constant of [11C]raclopride from plasma to brain) for the 18 subjects for whom k1 was available did not show an age effect for the striatum (r=0.31, df=16, p<0.20) or for the cerebellum (r=0.20, df=16, p=0.44). In contrast, the distribution volume measures for [11C]raclopride in the striatum were found to decrease significantly with age (figure 1). The significant correlations between D2 receptor availability and age were in the caudate (r=–0.62, df=28, p<0.0003) and the putamen (r=–0.70, df=28, p<0.0001).
Significant correlations between D2 receptor availability and results of neuropsychological tests were ubi~quitous. However, as expected, neuropsychological test performance also correlated with age for many of the tasks (table 1). Given the correlations between age and both dopamine and neuropsychological measures, partial correlations were calculated to control for age effects. The partial correlation analysis revealed specific relationships between dopamine measures and performance on the dopamine-sensitive tests. Partial correlations with D2 receptor availability were significant for the motor task (Finger Tapping Test) both in the caudate (r=0.56, df=27, p<0.005) and in the putamen (r=0.56, df=27, p<0.005) (figure 2); they were significant for the Wisconsin Card Sorting Test (total categories) (r=–0.42, df=27, p<0.05), the Stroop Color-Word Test (interference score) (r=0.38, df=27, p<0.05), the Raven Standard Progressive Matrices (r=0.37, df=27, p<0.05), and the Symbol Digit Modalities Test (written) (r=0.38, df=27, p<0.05) in the caudate only (table 2).
Years of education did not correlate with D2 receptor measures (caudate: r=0.20, df=28, p=0.32; putamen: r=0.25, df=28, p=0.21) or with the subject's age (r=0.11, df=28, p=0.60). The only neuropsychological measure for which there tended to be a correlation with years of education was the Wisconsin Card Sorting Test (total categories) (r=0.36, df=28, p=0.06).
The factor analysis of the neuropsychological test results revealed five factors, three of which contributed to 68% of the variance. Factor 1 comprised significant weights for the Stroop Color-Word Test (word=0.80, color=0.72, and interference=0.53) and the Symbol Digit Modalities Test (written=0.80, and oral=0.76). Factor 2 comprised significant weights for the Wisconsin Card Sorting Test (categories=0.77, errors=0.97, and perseverations=0.94). Factor 3 comprised a significant weight in the Finger Tapping Test (0.71). The tests in these three factors were the ones that were also found to be significantly correlated with the dopamine D2 receptor measures, corroborating a functional categorization of these tests.
Stepwise regression analysis with the 20 neuropsychological test scores showed that the Stroop Color-Word Test and the Finger Tapping Test accounted for most of the variance in dopamine D2 measures. The first step regression showed that the Stroop Color-Word Test contributed the most to the variance (R=0.72, F=20.7, df=1,19, p<0.0002), and the second step analysis selected the Finger Tapping Test (R=0.79, F=15.0, df=2,18, p<0.0001).
DISCUSSION
This study shows that the reductions in brain dopamine activity that occur as part of aging are associated with changes in motor as well as cognitive functions. An association between parameters of dopamine brain function and cognitive and motor performance had previously been shown in patients with Parkinson's disease (30, 31). The fact that this association is observed in healthy subjects with no evidence of neurological dysfunction indicates that while the decline in brain dopamine function may be clinically asymptomatic, it nonetheless leads to a decline in the individual's performance of specific cognitive and motor tasks.
The strongest association with D2 receptors was with the motor task (Finger Tapping Test), which is consistent with the well-recognized role of dopamine in motor control and regulation (32). While 80% of brain dopamine has to be lost for symptoms of Parkinson's disease to emerge (33), the results from this study indicate that smaller losses have an effect on motor behavior. Thus, the sensitivity of the Finger Tapping Test to age effects (34) is likely to reflect in part a decline in dopamine brain function. The relatively strong association of the Finger Tapping Test results with D2 receptors indicates that the test may be particularly useful for indirectly monitoring dopamine brain function.
This study also documents an association between dopamine measures and performance on behavioral measures of frontal lobe functioning—more specifically, tests of executive function requiring abstraction and mental flexibility (Wisconsin Card Sorting Test) and attention and response inhibition (Stroop Color-Word Test). Dopamine modulation of frontal activity during the performance of these tasks has been demonstrated both for the Stroop Color-Word Test (35) and for the Wisconsin Card Sorting Test (36). Imaging studies have also documented a decline in frontal metabolism with age (37, 38) and have shown an association between dopamine D2 receptor measures and metabolic activity in frontal brain regions (39). Thus, our results serve to further corroborate an involvement of the dopamine system in the cognitive deficits of the elderly that involve predominantly frontal lobe functions and that have been shown to be ameliorated by dopamine-enhancing drugs (40). Furthermore, the fact that correlations with neuropsychological performance remained significant after the partialing out of age effects suggests that dopamine activity may influence cognitive performance involving frontal lobe function irrespective of age.
In analyzing the results from this investigation, it is also useful to compare them with findings in patients with degeneration of the dopamine system, such as those with idiopathic Parkinson's disease and parkinsonism from exposure to MPTP. In particular, subjects exposed to MPTP provide a good model for assessing the consequences of changes in dopamine function. Unlike patients with Parkinson's disease, in which multiple neurotransmitters appear to be involved, patients with MPTP exposure show a selective lesion of the substantia nigra pars compacta (22, 23). These patients show impairments on the Wisconsin Card Sorting Test as well as on the Stroop Color-Word Test that have been referred to as a disturbance in executive functions or “the planning and modulation of ongoing behavior” (22, 23). Results on these two tests were also the ones that we found to be significantly correlated with dopamine activity in our healthy volunteers, corroborating the involvement of the dopamine system in cerebral functions involved in the performance of these tests. Subjects exposed to MPTP also have shown impairments in tasks that we did not find to be correlated with dopamine parameters, including tests for verbal fluency such as the Controlled Oral Word Association Test and tests of construction such as the Benton Visual Retention Test. Failure to document an association between dopamine parameters and these latter tests in our healthy subjects could indicate that changes in brain function may be different when decline in dopamine function occurs slowly, as with normal aging, than when it occurs abruptly, as in subjects exposed to MPTP. A gradual decline may enable the development of compensatory strategies for problem solving. In this respect, it is noteworthy that if patients with Parkinson's disease are assisted with the executive aspects of a construction task, their performance is improved (22).
The implications of these results are relevant to treatment, since they indicate that if one can identify strategies to improve dopamine brain function, these interventions could improve motor and cognitive performance in elderly subjects. For example, in laboratory animals dietary restriction retarded the age-related loss of dopamine D2 receptors (32), and changes in motor activity from a sedentary to an exercise-rich lifestyle led to increases in D2 receptors (41, 42). Thus, one could evaluate whether, for example, the improved performance on the Stroop Color-Word Test observed with exercise in sedentary humans (50–70 years of age) (43) is in fact associated with changes in D2 receptors and ultimately target lifestyles as well as medications that favor an active dopamine system.
The results of this study are also relevant for an understanding of the cognitive abnormalities reported in patients suffering from disease with dopamine involvement, such as Parkinson's disease and schizophrenia, whose impaired performance has been documented on both the Wisconsin Card Sorting Test and the Stroop Color-Word Test (44, 45).
A limitation in the interpretation of these data is that one cannot determine whether the decline in dopamine D2 receptors represents cell loss and/or a decline in the expression of D2 receptors in striatal cells. Thus, one cannot determine whether decline in D2 receptors with age represents primary or secondary changes in the striatum. This is particularly relevant, since imaging studies have shown changes in the striatum with aging. More specifically, MRI studies have shown an increase in T2 high signal intensity lesions with age, which is most prevalent in the striatum (46), and PET metabolic studies have shown a relative increase in striatal versus frontal metabolic activity in the elderly (38). Furthermore, the striatal changes may have functional consequences, as evidenced by a reported correlation between the presence of T2 high signal intensity lesions and performance on the Benton Visual Retention Test (46). However, it is unlikely that decline in D2 receptors with age reflects only nonspecific tissue loss in the striatum, since k1 measures in the striatum, which reflect cerebral blood flow, did not decline with age as would have been expected if the decline in D2 receptors were due solely to tissue loss.
Although we did not use MRI coregistration to select the regions of interest in this study, we have shown comparable estimates for dopamine D2 receptor measures when regions are selected directly from the PET images and when they are coregistered with MRI and then projected onto the PET images (26). To minimize quantification errors from possible effects of striatal atrophy with aging, the size of the regions of interest selected was less than half the size of the structures sampled in all directions, and the regions were placed in the center of the structure. A limitation in the generalizability of these data is that the education and verbal IQ of this study group were above average, and hence these findings require replication in groups more representative of the general population.
For many years neuropsychologists have struggled with developing test batteries that would probe specific functional brain circuits. The availability of imaging methods offers the possibility of determining not only the neuroanatomical brain structures involved with neuropsychological test performance but also the role that specific neurotransmitters may play in the mechanisms through which brain circuits interact to perform cerebral functions. In this study we selected neuropsychological tests that we presumed had a major dopaminergic component, since they show abnormal results in patients with Parkinson's disease. However, this assumption is limited by the fact that Parkinson's disease is likely to involve multiple neurotransmitters. Thus, the demonstration of a significant correlation between performance of those tasks and dopamine D2 measures serves to corroborate an important role of dopamine in the processing of the tasks in healthy individuals.
In summary, this study documents an association between the age-related decline in dopamine brain function and impaired performance on specific motor and cognitive tasks in healthy individuals. It also corroborates an involvement of dopamine in cognitive tests of executive function in healthy individuals. These results support the need to investigate interventions that enhance dopamine function to improve motor and cognitive performance and enhance the quality of life of the elderly.
 |
 |
Presented in part at the 33rd annual meeting of the American College of Neuropsychopharmacology, San Juan, Puerto Rico, Dec. 12–16, 1994. Received April 9, 1997; revisions received Sept. 8 and Oct. 8, 1997; accepted Oct. 13, 1997. From the Medical and Chemistry Departments, Brookhaven National Laboratory; the Department of Psychiatry, State University of New York at Stony Brook; and the Brain-Behavior Laboratory, Department of Psychiatry, University of Pennsylvania School of Medicine, Philadelphia. Address reprint requests to Dr. Volkow, Brookhaven National Laboratory, Upton, NY 11973; [email protected] (e-mail). Supported in part by the U.S. Department of Energy under contract DE-ACO2-76CH00016 and grant DA-06891 from the National Institute on Drug Abuse. The authors thank David Schlyer for cyclotron operations; Alex Levy, Naomi Pappas, and Donald Warner for PET operations; Colleen Shea, Richard Ferrieri, Robert MacGregor, and Payton King for radiotracer preparation; Noelwah Netusil for care of subjects; Cristopher Wong for data management; Carol Redvanly for organization; and Laurence Tancredi and Alfred Wolf for review and discussion.
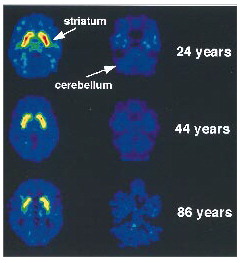
FIGURE 1. aNote the reductions in dopamine D2 receptors in the striatum as a function of age
Distribution Volume Images for the Dopamine D2 Radiotracer [11C]Raclopride at the Level of the Striatum and the Cerebellum in Three Subjects Aged 24, 44, and 86 Yearsa
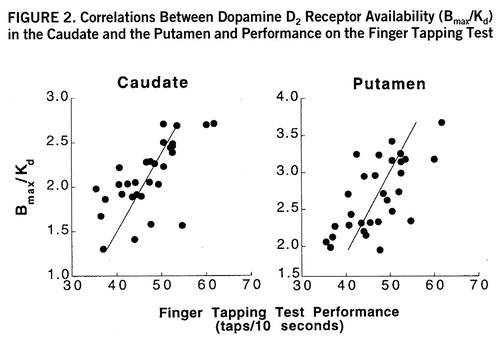
FIGURE 2. Correlations Between Dopamine D2 Receptor Availability (Bmax/Kd) in the Caudate and the Putamen and Performance on the Finger Tapping Test
1. Joseph JA, Roth GS, Strong R: The striatum, a microcosm for the examination of age-related alterations in the CNS: a selected review. Rev Biol Res Aging 1990; 4:181–199Google Scholar
2. Pradhan SN: Central neurotransmitters and aging. Life Sci 1980; 26:1643–1656Google Scholar
3. Morris JC, Storandt M, McKeel DW Jr, Rubin EH, Price JL, Berg L: Cerebral amyloid deposition and diffuse plaques in “normal” aging: evidence of presymptomatic and very mild Alzheimer's disease. Neurology 1996; 46:707–719Crossref, Medline, Google Scholar
4. Volkow ND, Fowler JS, Gatley JS, Logan J, Wang G-J, Ding Y-S, Dewey SL: Evaluation of the human brain dopamine system with PET: review and update of tracers. J Nucl Med 1996; 37:1242–1256Google Scholar
5. Volkow ND, Wang G-J, Fowler JS, Logan J, Gatley JS, MacGregor RR, Schlyer D, Hitzemann R, Wolf AP: Measuring age-related changes in DA D2 receptors with [11C]raclopride and with [18F]N-methylspiroperidol. Psychiatry Res 1996; 67:11–16Crossref, Medline, Google Scholar
6. Suhara T, Fukuda H, Inoue O, Itoh T, Suzuki K, Yamasaki T, Tateno Y: Age-related changes in human D1 dopamine receptors measured by positron emission tomography. Psychopharmacology (Berl) 1991; 103:41–45Crossref, Medline, Google Scholar
7. Volkow ND, Ding Y-S, Fowler JS, Wang G-J, Logan J, Gatley SJ, Hitzemann R, Smith G, Fields F, Gur R, Wolf AP: Dopamine transporters decrease with age in healthy subjects. J Nucl Med 1996; 37:554–558Medline, Google Scholar
8. Eidelberg D, Takikawa S, Dhawan V, Chaly T, Robeson W, Dahl R, Margouleff D, Moeller JR, Patlak CS, Fahn S: Striatal 18F-DOPA uptake: absence of an aging effect. J Cereb Blood Flow Metab 1993; 13:881–888Crossref, Medline, Google Scholar
9. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
10. Berg EA: A simple objective test for measuring flexibility in thinking. J Geriatric Psychol 1948; 39:15–22Google Scholar
11. Stroop JR: Studies of interference in serial verbal reactions. J Exp Psychol 1935; 18:643–662Crossref, Google Scholar
12. Reitan R: Validity of the Trail Making Test as an indication of organic brain damage. Percept Mot Skills 1958; 8:271–276Crossref, Google Scholar
13. Spreen O, Strauss E: A Compendium of Neuropsychological Tests. New York, Oxford University Press, 1991Google Scholar
14. Talland GA, Schwab RS: Performance with multiple sets in Parkinson's disease. Neuropsychologia 1964; 2:45–53Crossref, Google Scholar
15. Smith A: The Symbol Digit Modalities Test: a neuropsychologic test for economic screening of learning and other cerebral disorders. Learning Disorders 1968; 3:83–91Google Scholar
16. Raven JC: Guide to the Standard Progressive Matrices. London, HK Lewis, 1960Google Scholar
17. DeFillipis NA, McCampbell E, Rogers P: Development of a booklet form of the Category Test: normative and validity data. J Clin Neuropsychol 1979; 1:339–342Crossref, Google Scholar
18. Wechsler D: Wechsler Adult Intelligence Scale—Revised: Manual. New York, Psychological Corp, 1981Google Scholar
19. Jastak JF, Jastak SR: The Wide Range Achievement Test—Revised: Manual. Wilmington, Del, Guidance Associates, 1978Google Scholar
20. Wechsler D: Wechsler Memory Scale—Revised: Manual. New York, Psychological Corp, 1987Google Scholar
21. Benton AL: The Revised Visual Retention Test, 4th ed. New York, Psychological Corp, 1974Google Scholar
22. Stern Y, Langston J: Intellectual changes in patients with MPTP-induced parkinsonism. Neurology 1985; 35:1506–1509Google Scholar
23. Stern Y, Tetraud J, Martin W, Kutner S, Langston J: Cognitive change following MPTP exposure. Neurology 1990; 40:261–264Crossref, Medline, Google Scholar
24. Blonder LX, Gur RE, Gur RC, Saykin AJ, Hurtig HI: Neuropsychological functioning in hemiparkinsonism. Brain Cogn 1989; 9:177–190Crossref, Google Scholar
25. Volkow ND, Fowler JS, Wang G-J, Dewey SL, Schlyer D, Mac~Gregor R, Logan J, Alexoff D, Shea C, Hitzemann R, Angrist B, Wolf AP: Reproducibility of repeated measures of 11C raclopride binding in the human brain. J Nucl Med 1993; 34:609–613Medline, Google Scholar
26. Wang G-J, Volkow ND, Levy AV, Fowler JS, Logan J, Alexoff D, Hitzemann RJ, Schlyer D: MR-PET image coregistration for quantitation of striatal dopamine D2 receptors. J Comput Assist Tomogr 1996; 20:423–428Crossref, Medline, Google Scholar
27. Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer D, MacGregor RR, Hitzemann R, Bendriem B, Gatley SJ, Christman DR: Graphical analysis of reversible binding from time-activity measurements. J Cereb Blood Flow Metab 1990; 10:740–747Crossref, Medline, Google Scholar
28. Logan J, Volkow ND, Fowler JS, Wang G-J, Dewey SL, MacGregor R, Schlyer D, Gatley SJ, Pappas N, King P, Hitzemann R, Vitkun S: Effects of blood flow on [11C]raclopride binding in the brain: model simulations and kinetic analysis of PET data. J Cereb Blood Flow Metab 1994; 14:995–1010Google Scholar
29. Saykin AJ, Gur RC, Gur RE, Shtasel DL, Flanery KA, Mozley LH, Malamud BL, Watson B, Mozley PD: Normative neuropsychological test performance: effects of age, education, gender and ethnicity. Applied Neuropsychology 1995; 2:79–88Crossref, Medline, Google Scholar
30. Brooks DJ, Salmon EP, Mathias CJ: The relationship between locomotor disability, autonomic dysfunction, and the integrity of the striatal dopaminergic system in patients with multiple system atrophy, pure autonomic failure, and Parkinson's disease: studies with positron emission tomography. Brain 1990; 113:1539–1552Google Scholar
31. Holthoff VA, Kessler J, Pietrzyk U, Bonner J, Herholz K, Wienhard K, Wagner R, Vieregge P, Heiss W-D: Motor and cognitive deficits in Parkinson's disease are related to striatal dopamine uptake (abstract). J Cereb Blood Flow Metab 1993; 13:S253Google Scholar
32. Levin P, Janda JK, Joseph JA, Ingram DK, Roth GS: Peculiarities of the effect of hormones and transmitters during aging: modulation of changes in dopaminergic actions. Gerontology 1988; 34:22–28Crossref, Medline, Google Scholar
33. Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Sei~telberger F: Brain dopamine and the syndromes of Parkinson and Huntington: clinical, morphological and neurochemical correlations. J Neurol Sci 1973; 20:415–455Crossref, Medline, Google Scholar
34. Heaton RK, Grant I, Matthews CG: Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographic Corrections, Research Findings, and Clinical Applications. Odessa, Fla, Psychological Assessment Resources, 1991Google Scholar
35. Dolan RJ, Fletcher P, Frith CD, Friston KJ, Frackowiak RSJ, Grasby PM: Dopaminergic modulation of impaired cognitive activation in the anterior cingulate cortex in schizophrenia. Nature 1995; 378:180–182Crossref, Medline, Google Scholar
36. Daniel DG, Weinberger DR, Jones DW, Zigun JR, Coppola R, Handel S, Bigelow LB, Goldberg TE, Berman KF, Kleinman JE: The effect of amphetamine on regional cerebral blood flow during cognitive activation in schizophrenia. J Neurosci 1991; 11:1907–1917Google Scholar
37. Gur RC, Gur RE, Obrist WD, Skolnick BE, Reivich M: Age and regional cerebral blood flow at rest and during cognitive activity. Arch Gen Psychiatry 1987; 44:617–621Crossref, Medline, Google Scholar
38. Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Mande F, Alexander GE, Grady C, Pietrini P, Eidelberg D: The metabolic topography of normal aging. J Cereb Blood Flow Metab 1996; 16:385–398Crossref, Medline, Google Scholar
39. Volkow ND, Fowler JS, Wang G-J, Hitzemann R, Logan J, Schlyer D, Dewey S, Wolf AP: Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 1993; 14:169–177Crossref, Medline, Google Scholar
40. Ollat H: Dopaminergic insufficiency reflecting cerebral ageing: value of a dopaminergic agonist, piribedil. J Neurol 1992; 239(suppl 1):S13–S16Google Scholar
41. DeCastro JM, Duncan G: Operantly conditioned running: effects on brain catecholamine concentrations and receptor densities in the rat. Biochem Behav 1985; 23:495–500Crossref, Google Scholar
42. Gilliam PE, Spiduso WW, Martin TP, Walters TJ, Wilcox RE, Farrer RP: The effects of exercise training on 3H-spiperone binding in rat striatum. Pharmacol Biochem Behav 1984; 20:863–867Crossref, Medline, Google Scholar
43. Dustman RE, Ruhling RO, Russell EM, Shearer DE, Bonekat HW, Shigeoka JW, Wood JS, Bradford DC: Aerobic exercise training and improved neuropsychological function of older individuals. Neurobiol Aging 1984; 5:35–42Crossref, Medline, Google Scholar
44. Cohen JD, Schreiber DS: Context, cortex and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychol Rev 1992; 99:45–77Crossref, Medline, Google Scholar
45. Bowen FP, Kamienny RS, Burns MM, Yahr MD: Parkinsonism: effects of L-dopa treatment on concept formation. Neurology 1975; 25:701–704Crossref, Medline, Google Scholar
46. Kasahara H, Yamada H, Tanno M, Kobayashi M, Karasawa A, Endo K, Ushijima S: Magnetic resonance imaging study of the brain in aged volunteers: T2 high intensity lesions and higher order cortical function. Psychiatry Clin Neurosci 1995; 49:273–279Crossref, Medline, Google Scholar



