Schizophrenia-Like Psychosis and Epilepsy: The Status of the Association
Abstract
OBJECTIVE: Current knowledge of the relationship between epilepsy and schizophrenia-like psychosis is examined, and the proposed pathogenetic mechanisms are evaluated. METHOD: The author provides an overview of the published literature on epilepsy and schizophrenia-like psychosis. RESULTS: The schizophrenia-like psychoses of epilepsy are inadequately categorized by the current classifications. Their categorization into ictal, postictal, and interictal psychoses is clinically useful, but it does not imply distinct pathophysiology for each. The recent interest in postictal psychoses has opened an important avenue for research. Brief interictal psychoses, involving alternation between epilepsy and psychosis and accompanied by forced normalization, are uncommon. Many aspects of the relationship with chronic interictal psychosis remain controversial. The majority of investigators support a special but not exclusive relationship with mediobasal temporal lobe epilepsy, and left temporal bias receives only limited support. The chronic psychosis resembles schizophrenia phenomenologically. Some suggested risk factors are severe and intractable epilepsy, epilepsy of early onset, secondary generalization of seizures, certain anticonvulsant drugs, and temporal lobectomy. Different neuropathological studies suggest the presence of cortical dysgenesis or diffuse brain damage. CONCLUSIONS: There are many mechanisms by which epilepsy may be associated with schizophrenia-like psychosis. It is likely that structural brain abnormalities, e.g., cortical dysgenesis or diffuse brain lesions, underlie both epilepsy and psychosis, and that the seizures modify the presentation of the psychosis, and vice versa, thus producing a clinical picture of both an affinity and an antagonism between the two disorders.
The association between epilepsy and schizophrenia-like psychosis has attracted the attention of psychiatrists since the nineteenth century, but many aspects of this relationship still remain controversial (1–5). Recent developments in the understanding of the neurobiology of epilepsy and schizophrenia prompted a reexamination of the association. In the past, such an examination has been constrained by data and conceptual limitations, some of which are beginning to be remedied.
LIMITATIONS OF PREVIOUS ANALYSES
First, the definitions of “psychosis” and “schizophrenia” used in many studies have lacked standardization. Clearly, the significance of a confusional postictal psychosis is different from that of a postictal manic psychosis or an interictal schizophreniform psychosis. In this review, I restrict myself to the examination of psychoses that phenomenologically resemble a schizophreniform illness.
Second, both epilepsy and schizophrenia are heterogeneous disorders, and their categorization should be systematically studied before any association is described. For example, estimates of the proportion of epilepsy patients who have a temporal lobe focus vary from 30% to as much as 76% (6), and studies have differed in the rigor with which a temporal lobe onset was investigated. The 6-month criterion for schizophrenia used in DSM-III and subsequent classifications had a major impact on the prevalence rates for this disorder, with implications for associations based on previous epidemiological data.
Third, the similarity between temporal lobe phenomena and psychotic symptoms does not necessarily imply a common origin for the two sets. Similar brain phenomena may be produced by pathology in different brain regions (7). The exclusive focus on seizures in the epileptic patient may also be in error as the epileptic brain is not normal between seizures. Even when neuroimaging or interictal EEG abnormalities are lacking, some abnormality at the cellular or molecular level is likely to be present (8), and the psychosis may be related to that underlying abnormality. Furthermore, epilepsy is not a static process, and the brain of the epileptic patient is undergoing structural and neurochemical change before and after the development of seizures, to which ictal events actually contribute (9).
Fourth, the “affinity” and “antagonism” hypotheses of epilepsy and schizophrenia-like psychosis, which may seem mutually inconsistent on the surface, have to be reconciled in any analysis. The two themes that have dominated psychiatric thought on the association between schizophrenia-like psychosis and epilepsy are 1) they occur together more often than by chance (10), and 2) they are antagonistic to each other (11). The latter was the primary motivation for the introduction of convulsive therapy for the treatment of schizophrenia. Since the evidence suggests that both associations are possible, at least in a modified form, the paradox must be addressed if not resolved.
CATEGORIZATION
A consensus on the classification of psychotic syndromes associated with epilepsy is lacking, and neither DSM-IV nor ICD-10 has addressed this issue specifically. Application of DSM-IV criteria results in an ambiguous situation in which one can make the diagnosis of a secondary (due to a general medical condition) or primary psychotic syndrome depending on whether the nature of the evidence prompts one to deduce that the psychotic disturbance is etiologically related to the epilepsy “through a physiological mechanism” (p. 307). Most often, separate diagnoses of epilepsy and the particular psychotic syndrome are appropriate. In such circumstances, note should be made of the relationship of the psychosis with the onset of epilepsy, seizure frequency, recent seizure episodes, current anticonvulsant medication, EEG abnormalities, and the underlying neurological lesion, if known.
Since clinical seizures are the outstanding feature of epilepsy, psychotic syndromes have traditionally been classified according to their temporal relationship to these events, as ictal, postictal (or peri-ictal), and interictal, with the last type being either brief or chronic. For this review, I will retain this classification without implying that these categories are distinct in their pathophysiology.
ICTAL PSYCHOSIS
A nonconvulsive status epilepticus can result in symptoms resembling psychosis, even though the appropriate DSM-IV diagnosis would usually be delirium. The psychosis is necessarily brief, usually hours to days. When prolonged into days, it is likely to be ictal behavior that extends postictally. The most common association is with partial complex (or psychomotor) status, and patients may present a wide range of perceptual, behavioral, cognitive, and affective symptoms, often in association with automatisms involving oral activity, picking at clothes, and paucity of speech or mutism (12, 13). Consciousness is altered during the episode but may be difficult to test, and patients are amnesic for the episode. Simple partial status may produce affective, autonomic, and psychic symptoms that may include hallucinations and thought disorder in clear consciousness. Insight is usually maintained, and the manifestation is not that of a true psychosis, but the symptoms may be misinterpreted or embellished by the patient and behavioral disturbance may result (14). Petit mal status (absence or spike-wave status) results in altered consciousness and such motor symptoms as eyelid fluttering and myoclonic jerks, and it may superficially resemble psychosis with disorganized behavior, but delusions and hallucinations are lacking (13). Patients with ictal psychosis usually have a history of epilepsy.
By definition, ictal psychosis is concurrently associated with epileptic discharges in the brain, and except in some patients with simple partial status (15), scalp EEG abnormalities are detectable. The majority of discharges have a focus in the limbic and isocortical components of the temporal lobe, but the focus is extratemporal in about 30% of patients (16), usually in the frontal or cingulate cortex. Since the scalp EEG may be normal in simple partial seizures, the behavioral disturbance may be mistaken to be interictal, and a high index of suspicion is necessary. Resolution of the disturbance with intravenous diazepam is not diagnostically foolproof as many nonepileptic behaviors may so resolve (14).
Symptoms may reflect one of two mechanisms (17): 1) a positive effect of the seizure discharge, i.e., the epileptic discharge activates a behavioral mechanism represented in the area subjected to the discharge, and 2) a negative effect, i.e., either a) the individual is unable to engage in a certain behavior owing to the temporary paralysis of the anatomical substrate of that behavior or b) some behaviors are released by the inactivation of structures that normally suppress them. Behavioral disturbance due to a negative effect may occur in other situations, e.g., when the whole cerebral cortex is subjected to a relatively mild form of seizure activity represented by generalized spike and wave discharges. This negative effect may continue postictally, or it may initiate then. Experiential phenomena in ictal psychosis are likely to be due to positive effects, whereas automatisms may be caused by positive or negative effects.
The question of whether chronic psychoses in clear consciousness can be a direct consequence of continuous seizure activity restricted to deep brain structures has generated much controversy. Most epileptologists consider this to be extremely unlikely (14), but this long-held idea remains current (2). Kendrick and Gibbs (18) first used implanted electrodes to study the electrophysiological disturbance in schizophrenia and in the psychoses of psychomotor epilepsy, and spike discharges in medial temporal and frontal structures were demonstrated in both patient groups. Kendrick and Gibbs reported that surgery on medial temporal structures was nearly always beneficial for schizophrenia. Sem-Jacobsen (19) and Heath (20) noted similar abnormal discharges that did not spread beyond the amygdala, hippocampus, and septal regions, again in both schizophrenic and “epileptic psychosis” patients. Scalp EEGs were normal or only mildly abnormal. These findings have not been further examined, perhaps owing to ethical constraints against using depth recordings for research purposes. A noninvasive neurophysiological technique that may be able to detect deep limbic discharges is magnetoencephalography, arguably an important tool for future attempts to address this issue (21).
Even if continuous ictal activity in depth recordings can be demonstrated, appropriate longitudinal and controlled studies are necessary to establish its significance. First, epileptiform activity in deep structures may be common in nonpsychotic patients with focal epilepsy, especially in the postictal period (22). Second, rapid synchronized neuronal discharges, which form the basis of epileptic discharges, occur normally in the limbic structures, hypothalamus, and brainstem in association with vital functions such as parturition, milk ejection, growth hormone release, and orgasm (4).
POSTICTAL PSYCHOSIS
That brief psychotic episodes may follow a bout of seizures has received much attention in the last decade (23–29). Since they are brief and occur in close proximity with seizures, they are ideal for the investigation of some pathogenetic mechanisms. They usually follow seizure clusters or a recent exacerbation in seizure frequency (23) that may be related to withdrawal of anticonvulsants, often as a part of the video-EEG monitoring of patients (25, 27). Postictal psychoses are common in epilepsy-monitoring facilities; 6.4% of the patients in one study developed this syndrome (27), and nearly 10% did so in another study (28). If the psychosis develops gradually and in parallel with increasing seizure frequency, it may be referred to as peri-ictal rather than postictal, but there is no reason to believe that this distinction is meaningful clinically or for its pathophysiology.
Between the last seizure and the psychosis there is usually a nonpsychotic period, which ranges from a few hours to a few days. It was 12–72 hours in the Kanner et al. study (27) and up to 1 week according to Logsdail and Toone (23). Some clouding of consciousness is often present in this period, and it may extend to the initial period of psychosis or even the whole episode. The psychotic symptoms are pleomorphic (persecutory, grandiose, referential, somatic, and religious delusions, catatonia, hallucinations, etc.), and affective symptoms (manic or depressive) are often prominent (23, 29). First-rank symptoms of Schneider can occur but are rare (27). Postictal psychoses resolve within a few days, with the mean duration in the study by Kanner et al. (27) being about 70 hours (range=24–144), and all resolving within 1 month in the study by Savard et al. (25). Resolution is aided by neuroleptic medication, usually in small doses. A further seizure may exacerbate the psychosis, and anticonvulsant treatment should be carefully monitored. The brief psychosis may recur, at a frequency of 2–3 episodes per year in two studies (27, 30), and in some patients—15% in one study (23)—these episodes may become chronic.
The predisposing factors are poorly understood. The majority of patients suffer from partial complex seizures that are secondarily generalized. Epilepsy has often been present for more than 10 years before the onset of psychosis (23, 27, 28). EEG abnormalities persist in the majority during the psychosis (23). In a case report by So et al. (24), the patient with postictal psychosis demonstrated frequent bitemporal independent epileptiform discharges on depth recording that were maximal in the mesial limbic regions. While Kanner et al. (27) found no specific predisposing factors that differentiated their psychotic group from a comparable nonpsychotic epilepsy group, Savard et al. (25) were impressed with a high rate of ictal fear, bilateral independent discharges, and gross structural lesions (six out of nine patients), including the presence of alien tissue tumors. Kanemoto et al. (29) also noted frequent psychic auras, and Umbricht et al. (31) noted frequent bitemporal foci in their subjects. Five of the 14 patients studied by Logsdail and Toone (23) had abnormalities on brain computerized tomography (CT). In the magnetic resonance imaging (MRI) study of Kanemoto et al. (28), postictal psychosis was most likely to occur in patients with resistant temporal lobe epilepsy stemming from mesial temporal sclerosis, especially on the left side. These patients with left-side mesial temporal sclerosis were also likely to have atrophy of the temporal neocortex.
The pathogenetic mechanisms are poorly understood. The finding of chronic frequent subictal discharges suggests that ictal activity in the temporal lobe is directly related to this kind of psychosis. Changes in monoamines, particularly postsynaptic dopamine receptor sensitivity, have been suggested as the mediating mechanism (24). Some support for the dopamine mechanism came from a single photon emission computed tomography (SPECT) study using [123I]iodobenzamide that demonstrated low levels of striatal dopamine D2 receptors in patients with peri-ictal psychosis (32). Seizures also lead to a number of other neurochemical changes, as part of homeostatic mechanisms, which may play a role in the pathogenesis of the psychosis: increase in turnover of γ-aminobutyric acid (GABA), reduction in cerebral concentrations of aspartate and glutamate, and changes in endorphins, peptides, brain adenosine, and the second messenger system (33). Low folic acid levels may have a role (34), but firm evidence is lacking. The significance of a report of hyponatremia in these patients is also unknown (27). Postictal psychosis has also been conceptualized as a phenomenon akin to Todd's paralysis, indicating the postictal inactivation of cortical regions involved in the ictal event, which usually include bilateral medial temporal structures (14). A SPECT study of four patients with temporal lobe epilepsy and postictal psychosis demonstrated mesial frontal hyperperfusion during the psychosis (26). Patients with such psychoses would be ideal candidates for longitudinal studies.
BRIEF INTERICTAL PSYCHOSIS
Brief psychotic episodes can also develop when seizures are infrequent or fully controlled. These psychoses last from days to weeks, they are usually self-limiting, and their separation from postictal psychoses may be difficult. The favored description is of an alternating psychosis (35), i.e., a brief psychosis alternating with periods of increased seizure activity such that the seizures and psychosis appear antagonistic. The phenomenology is characterized by paranoid delusions and auditory hallucinations, but multiple other features, including affective symptoms, may occur (35–38). Tellenbach (35) pointed out the presence of premonitory symptoms such as insomnia, anxiety, feelings of oppression, and withdrawal as heralding the psychosis, and Wolf (39) suggested that treatment with anxiolytics at this stage may prevent development of the psychosis. Unlike postictal psychosis, this psychosis can be ameliorated by the occurrence of one or more seizures (39).
The concept of forced normalization (forcierte Normalisierung) was introduced by Landolt (40) for the puzzling observation that the EEGs of epilepsy patients often looked less pathological when their behavior had deteriorated. This phenomenon, also called “paradoxical” or “spurious” normalization (39), has been documented by a number of authors (38, 39, 41) with the additional observations that 1) the EEG may become more, rather than entirely, normal; 2) the manifestation is not always of psychosis, and other disturbances, such as affective symptoms, an anxiety or dissociative state, and behavioral disturbance, may be present; and 3) not all brief interictal psychoses manifest this phenomenon (36). Alternating psychoses are uncommon, and Schmitz and Wolf (42) reported three cases of alternating psychosis in 697 epilepsy patients. Ramani and Gumnit (36) observed forced normalization in only one of nine epilepsy patients who became psychotic while being treated in the hospital for their epilepsy. Patients with brief interictal psychosis have been reported to suffer from either partial complex epilepsy or primary generalized epilepsy. While a temporal lobe onset is common, Wolf (39) argued that all of these patients also had generalized seizures. Kanemoto et al. (28) reported the frequent presence of mesial temporal sclerosis in patients with interictal psychosis, who also were likely to have experienced the onset of epilepsy before the age of 10 years.
Special relationships between brief interictal psychosis and two drug classes should be highlighted.
Psychosis and Anticonvulsant Drugs
Anticonvulsant drugs have been reported to precipitate psychosis, although the published literature is confounded by the inclusion of affective and confusional psychoses in this category. The facts that the control of seizures may induce psychosis in a few patients and that neuroleptic drugs, which are proconvulsant, are useful in treating such a psychosis are consistent with the concept of alternating psychosis and forced normalization. Gibbs (43) first drew attention to this in relation to phenacetylurea, and Landolt (44) implicated the succinimides. Wolf (39) further emphasized the relationship with ethosuximide and reported that valproate did not produce the same result. Others have reported psychosis in association with clobazam, phenytoin, carbamazepine, barbiturates, and benzodiazepines (1). Psychosis related to vigabatrin, a new antiepileptic drug that is an irreversible inhibitor of GABA aminotransferase, has excited much interest (45). Another group of drugs that are potent anticonvulsants in animal models is the N-methyl-D-aspartic acid (NMDA) antagonists, e.g., MK-801, ketamine, and phencyclidine, but these drugs are potent psychotogens (46), which limits their clinical utility.
Antipsychotic Drugs and Epilepsy
All antipsychotic drugs have the propensity to cause paroxysmal EEG abnormalities and induce seizures, and the effect is related to drug type and dose (47). The newer antipsychotics are not free of this effect, and in fact, clozapine is the most epileptogenic of the antipsychotics, with myoclonus or frank seizures reported in 0.3%–5% of patients treated with therapeutic doses (4).
CHRONIC INTERICTAL PSYCHOSIS
The investigation of the relationship between epilepsy and chronic schizophrenia-like psychosis was brought into the modern era by Slater et al. (10). Despite a great deal of further work, many aspects of this relationship remain controversial.
Evidence for Association
The epidemiological evidence for the association between epilepsy and chronic schizophrenia-like psychosis is summarized in table 1. The rates must be interpreted in light of the general prevalences of psychosis, schizophrenia, and epilepsy in the general population. Schizophrenia is estimated to have a prevalence of 0.5%–1% in the general population, but if we use a broad concept of psychosis, the prevalence is likely to be much higher (58). Epilepsy has a point prevalence of 0.4%–1% in the general population, and the lifetime risk of having at least one unprovoked seizure is 2%–5% (59, 60). The prevalence is low in the first decade of life, increases to a plateau in the adult years, and increases further in the elderly (60). Methodological difficulties in the studies should also be taken into account in the interpretation of the prevalence data. For example, the classic study by Slater et al. (10) drew its subjects from tertiary centers in two major London hospitals. The overall evidence suggests that schizophrenia-like psychosis is 6–12 times more likely to occur in epileptic patients than in the general population.
Is Greater Risk of Psychosis Particular to Temporal Lobe Epilepsy?
Suggestions that psychosis in epilepsy might be exclusively or preferentially associated with temporal lobe epilepsy are supported by the majority of investigations (10, 43, 61–63). Mendez et al. (3) reported a higher rate of partial complex seizures, but not temporal lobe foci, in their group with schizophrenia-like psychosis plus epilepsy than in their nonschizophrenic epilepsy comparison subjects. In studies that compared patients with temporal lobe epilepsy and those with generalized epilepsy (64, 65), the patients with temporal lobe epilepsy were more likely to be psychotic, but the subject groups in these studies were small. The major challenge has come from Stevens (58), who argued that the proportion of temporal lobe epilepsy in epilepsy-psychosis patients is no different from that in the adult epileptic population in general, the latter being estimated to be about 60% (59, 60). This debate, therefore, has not resolved but continues to be in favor of a special but not exclusive relationship between schizophrenia-like psychosis and temporal lobe epilepsy. Additionally, there are neuro~imaging and neuropathological data linking the temporal lobe with psychosis (discussed in later sections).
Mediobasal or Neocortical Temporal Lobe Epilepsy?
Kristensen and Sindrup (41) reported that psychotic patients had a substantial preponderance of temporal mediobasal spike foci, recorded on sphenoidal electrodes, and an excess of epigastric auras. Hermann et al. (66) reported a higher frequency of schizophrenia and other psychopathology in patients with an aura of fear. Mendez et al. (3) reported more psychic and autonomic auras in the psychotic patients. The neuropathological literature (see later section) has supported a predominant abnormality in the medial temporal structures, although more widespread damage has also been reported (67, 68). The majority of the evidence, therefore, points to a mediobasal rather than neocortical temporal lobe abnormality underpinning psychosis.
Laterality of Epileptic Focus?
Since the suggestion by Flor-Henry (69) of a preponderance of left-sided pathology in patients with schizophrenia-like psychosis, many studies have examined this issue. In the EEG studies, the majority opinion favors an excess of left temporal foci in the patients with temporal lobe epilepsy and schizophreniform psychosis (62, 70), although there have been some negative laterality studies (41, 65, 71). There are many problems with the available data. First, the rigor with which laterality was established differs across studies, and the use of surface EEG recordings to establish laterality is open to question. Second, the presence of an epileptic focus on one side does not mean that pathology is restricted to that side. Third, left-sided preponderance of temporal lobe foci may not be restricted to psychotic individuals, as the evidence supports a left-sided bias for temporal lobe epilepsy in general (49). Fourth, there is emerging evidence that epilepsy patients with schizophrenia have generalized seizures even when they have a temporal focus (3, 39). Fifth, the instruments and diagnostic criteria used for psychosis are language dependent, thus introducing a left-side bias (58).
The neuroimaging studies that examined laterality were inconclusive. The CT (63, 72) and MRI (73) studies failed to demonstrate lateralized lesions, although the patients with hallucinations had higher T1 values in the left temporal lobe. Two small functional imaging studies (74, 75) provided preliminary evidence of greater left medial temporal lobe dysfunction in schizophrenia-like psychosis with epilepsy. A proton magnetic resonance spectroscopy study (76) showed metabolic abnormalities in the left temporal lobe of patients with schizophrenia-like psychosis and epilepsy. The neuropathological studies (68, 77) have not supported lateralization of pathology.
The laterality issue therefore remains undecided, but the importance of a left-sided focus is not striking. It is possible that the structural abnormality in epileptic psychosis is not lateralized, and is possibly bilateral, but that the functional abnormality is predominantly left-sided. However, right-sided abnormality seems to be sufficient, and generalization of the epileptic disturbance is commonly present.
Clinical Presentation of Chronic Interictal Psychosis
Slater et al. (10) reported that they had found a mean age at onset of about 30 years and that the symptoms were largely paranoid-hallucinatory, commonly associated with catatonia, affective blunting, and volitional symptoms. Phenomenologically, the disorder was indistinguishable from schizophrenia, although the authors reported a better preservation of affect, mood swings, mystical experiences, and visual hallucinations. Investigators in two controlled studies from London (62, 63) also noted the largely paranoid-hallucinatory characteristics of the disorder, but they stressed the greater frequency of “organicity.” In the study by Mendez et al. (3), the epilepsy-with-schizophrenia group did not differ from the nonepileptic schizophrenic comparison subjects on any psychosis item except increased suicidal behavior. In conclusion, except for minor reported differences, which may be accounted for by selection biases in the comparison group, the chronic psychoses of epilepsy are similar to schizophrenia.
Many authors have commented on the relative lack of negative symptoms and a more benign course for epileptic schizophrenia (10, 72), but supportive controlled studies are lacking. Nearly one-half (45%) of the patients of Slater et al. (10) had chronic courses. In a 10-year follow-up study in Japan (78), 64% of the patients had chronic courses. This outcome may not be too different from that in schizophrenia with a relatively later age at onset.
Age at Onset, Duration, and Severity of Epilepsy
Epilepsy beginning at an early age (77) and enduring through puberty (57, 68, 79) has been associated with schizophrenia-like psychosis. Many years (usually 10–14) are said to intervene between the onsets of epilepsy and schizophrenia-like psychosis (10, 63, 72), but this period is highly variable and patients who develop epilepsy after the psychosis are usually excluded from such analyses. Moreover, the peak age at onset for epilepsy is in any case earlier than that for schizophrenia (59), thus making the relevance of this observation somewhat ambiguous.
It is often noted that the patients who develop psychosis have a severe form of epilepsy involving multiple seizure types (50), a history of status epilepticus (10), and resistance to drug treatment (5). The frequency of seizures at the time of development of the psychosis is variable; some authors report an improvement (57), whereas others report a worsening (3). Most often, it is not possible to relate the onset of the psychosis to any change in seizure frequency (10).
Other Risk Factors
A female sex bias was reported by one group (79) but is not generally supported (10, 63, 72). Patients who have schizophrenia-like psychosis with epilepsy generally have been reported not to have a greater than normal familial aggregation of schizophreniform disorders (10, 63, 72). Their premorbid personalities were normal in one study (10).
Postlobectomy Psychosis
Schizophrenia-like psychosis may develop de novo many months or years after temporal lobectomy for the treatment of intractable epilepsy. Rates from 3% to 28% have been reported, as summarized in table 2. The psychosis is usually a paranoid-hallucinatory state with depressive features (57, 71, 79), the neuropathology is diverse, the patients additionally have had generalized seizures, and there is an overrepresentation of right lobectomy (82–84). Roberts et al. (77) argued that the postoperative onset of psychosis may be an artifact of an earlier age at operation. There are some reports of improvement of schizophrenic symptoms with temporal lobectomy; it is interesting that these cases were associated with left-sided surgery (77, 83).
Neuroimaging Studies
A few neuroimaging studies must be highlighted in relation to chronic schizophrenia-like psychosis of epilepsy. An MRI study (73) already referred to did not show any difference in T1 relaxation times between epilepsy-psychosis patients and schizophrenic comparison subjects. There is an extensive literature on MRI brain morphometry of schizophrenia and temporal lobe epilepsy, and some of the morphological abnormalities described (large ventricles, small hippocampus) are common to the disorders (85). A positron emission tomography (PET) study using [15O]H2O demonstrated lower oxygen extraction ratios in the frontal, temporal, and basal ganglia regions of psychotic patients with epilepsy than in nonpsychotic epileptic patients (74), and a small study using SPECT showed lower left medial temporal blood flow in psychotic than nonpsychotic epileptic patients (75). The PET study of patients with psychosis by Reith et al. (86), which included two patients with chronic and two with postictal schizophrenia-like psychosis, showed higher than normal levels of dopa decarboxylase activity in schizophrenia-like psychosis and schizophrenia, and it was suggested to be due to suppressed tonic release of dopamine in striatum because of low corticostriatal glutamatergic input.
Neuropathological Studies
Neuropathological studies of schizophrenia-like psychosis with epilepsy have been limited. A large series from London, drawing on subjects with histories of temporal lobectomies, has been reported (77, 79). Taylor (79) commented that epilepsy patients with schizophrenia-like psychosis were less likely to have mesial temporal sclerosis and more likely to have alien tissue lesions (small tumors, hamartomas, and focal dysplasias). In the report by Roberts et al. (77), 40% of patients with schizophrenia-like psychosis and epilepsy had mesial temporal sclerosis, 20% had alien tissue gangliomas, and 20% had no lesions (49%, 4%, and 15% of the total epileptic group, respectively). Histories of birth injury, head injury, and febrile convulsions were not overrepresented in the group with schizophrenia-like psychosis, but the frequency of alien tissue tumors and early onset of seizures suggested a developmental lesion in the medial temporal structures that had been physiologically active from an early age. Stevens (67), on the other hand, reported widespread pathology in six cases of epilepsy and psychosis; the pathology included the hippocampus, hypothalamus, thalamus, pallidum, and cerebellum. In a study by Bruton et al. (68), epileptic patients with schizophrenia-like psychosis had larger ventricles, more periventricular gliosis, more focal damage, and more periventricular white matter softenings than nonpsychotic epileptic comparison subjects, but similar rates of mesial sclerosis, suggesting greater nonspecific neuropathology.
Possible Pathophysiological Mechanisms for Psychosis in Epilepsy
Discussion has centered broadly on two mechanisms: 1) the psychosis is due to the repeated electrical discharges, either directly or through the development of neurophysiological or neurochemical abnormalities, or 2) the epilepsy and psychosis share a common neuropathology that may be localized (emphasis on temporal lobe but also frontal lobe and the cerebellum) or widespread in the brain. Both mechanisms may be operative, the latter being primary and the former modifying the presentation, determining exacerbations and remissions, or being the proximate cause. The possible roles of psychological factors, neurotoxicity of anticonvulsant drugs, deficiencies (e.g., folic acid), and abnormal experiences seem to be of secondary importance.
Psychosis is a direct consequence of the epileptiform disturbance. I have previously referred to mechanisms by which seizures may directly result in ictal, postictal, and brief interictal psychoses: continuous subictal activity, homeostatic mechanisms that help reduce epileptic excitability, and neurochemical and neuroendocrine changes produced by the seizures. These explanations are inadequate for chronic schizophrenia-like psychosis even though they may account for some fluctuation of symptoms.
Kindling has been proposed as one possible mechanism for the occurrence of chronicity. Studies of behavioral and pharmacological kindling in animals, and the development of mirror foci, suggest that the potential exists for repetitive epileptiform discharges to facilitate subsequent propagation along specific pathways that may cause interictal disturbance. Electrical and pharmacological kindling of the ventral tegmentum and amygdaloid kindling by cocaine and apomorphine in the cat (87) have been suggested as model psychoses. The long duration of epilepsy before the onset of psychosis, the frequency of the partial seizures, and the limbic origin of the seizures provide evidence for this hypothesis. Kindling may thus explain some aspects of the relationship between epilepsy and psychosis, especially the antagonism (88), but there are limitations to the hypothesis: the relationship of the psychosis to seizures is variable in terms of age at onset, duration, and frequency; it is uncertain whether kindling can be permanent; patients usually have generalized seizures and widespread pathology; and kindling currently imputes the dopamine hypothesis of psychosis, support for which is inconsistent. Postlobectomy psychosis has been suggested to be a result of downstream kindling due to persistent ictal activity and perhaps decreased seizure frequency (83). Delayed psychosis after right temporoparietal stroke has been reported in one series of eight patients, seven of whom developed seizures before the psychosis (89).
Another mechanism by which frequent seizures may bring about chronic behavioral disturbance is the production of plastic regenerative changes affecting, in particular, the medial temporal lobes. It has been shown that stimulation of the hippocampus leads to an anomalous axonal sprouting from dentate granule cells before the development of seizures (90). Expansion of glutamatergic presynaptic mossy fibers and an increase in perforated postsynaptic densities on granule cell dendrites have been demonstrated in temporal lobectomy specimens (91), changes possibly produced by increased expression of messenger RNA for c-fos and NGF by recurrent limbic seizures. The aberrant regeneration and the resultant “miswiring,” alone or in combination with the underlying neuropathology, may be the underlying basis for chronic schizophrenia-like psychosis (see following section).
Both psychosis and epilepsy are symptomatic of an underlying neuropathological or physiological dysfunction. Two major possibilities have been suggested: 1) neuro~developmental abnormalities leading to cortical dysgenesis as the common factor and 2) diffuse brain damage causing both epilepsy and psychosis.
1. Cortical dysgenesis hypothesis. Neuropathological studies of temporal lobe epilepsy suggest that about two-thirds of the patients show hippocampal cell loss and sclerosis, particularly in the prosubiculum and CA1 regions, and a substantial proportion of temporal lobe epilepsy patients (92) have other pathologies, in particular gliomas, hamartomas, and heterotopias. The presence of these alien tissue lesions suggests defective neuroembryogenesis. Patients with mesial sclerosis commonly have heterotopias, hippocampal neuronal loss of about 20%–50% (92), and synaptic reorganization in the hippocampus. Veith (93) reported heterotopias in 37% of temporal lobe epilepsy patients but only 4% of nonepileptic comparison subjects. Heterotopic abnormalities have also been described in primary generalized epilepsies (94, 95). Cryptic insults such as childhood viruses, fever, or minor hypoxia may lead to synaptic reorganization in vulnerable brains, exacerbating the problem.
There is now considerable evidence that schizophrenia is associated with cortical maldevelopment (96). More than a decade ago it was demonstrated that schizophrenic patients had a disorganization of the pyramidal cell layer (97), which was thought to represent a problem in the migration of primitive neurons into the presumptive hippocampal plate. Heterotopias have been demonstrated in the brains of schizophrenic persons (98), and there is also evidence for synaptic reorganization (99). These disturbances either have a genetic basis or may be due to prenatal, perinatal, or early developmental insults.
If similar developmental abnormalities underlie both epilepsy and schizophrenia, it is not surprising that some epilepsy patients develop schizophrenia-like psychosis. The evidence that schizophrenia-like psychosis is more likely to develop in epileptic patients with developmental brain abnormalities (77, 79) is consistent with this suggestion. That epilepsy and psychosis have different ages at onset may be due to different functional consequences of the abnormalities, depending on the stage of neurodevelopment. Epileptic activity may, in addition, exacerbate an underlying dysgenesis, setting the stage for psychosis.
2. Diffuse brain damage hypothesis. While there has been extensive neuropathological interest in the temporal lobe, there is evidence that brain abnormalities in the brains of schizophrenic subjects are widespread (96). Most neuropathological studies of epilepsy and psychosis have been limited to the examination of resected temporal lobes. The studies by Stevens (83) and Bruton et al. (68) are exceptions, revealing excess pathology that was widespread and not dissimilar to some of the pathology reported for schizophrenia. These findings argue that schizophrenia-like psychosis may be related to degenerative or regenerative changes in the brain not directly related to the classic epileptic pathology.
3. Composite model. An attempt at a synthesis of the various hypotheses is presented in figure 1. According to this view, epilepsy patients who develop chronic schizophrenia-like psychosis have a brain lesion that makes them vulnerable to psychosis. This lesion may be neurodevelopmental, leading to cortical dysgenesis, or be acquired through trauma, hypoxia, infection, etc. The abnormality may be widespread but is particularly likely to involve the limbic structures, leading to abnormalities of connectivity of these structures to their afferent and efferent projection regions. Since the development of medial temporal structures is asymmetric, it may explain some of the laterality data. The abnormality is likely to cause electrical storms in the limbic cortex, with seizures occurring at an early age. The occurrence of frank seizures or microseizures exacerbates the abnormality owing to kindling mechanisms or the regenerative changes involving axonal sprouting and synaptic reorganization. In due course these result in disruption of anatomically distributed functional systems and lead to schizophrenia-like psychosis, accounting for the “affinity” between epilepsy and schizophrenia-like psychosis. Seizures, either by the presence of continuous subictal activity or by their modulation of catecholamine and pre- and postsynaptic glutamatergic and GABAergic activity, modulate the expression of the psychosis or act as brakes, sometimes leading to the impression of antagonism. The picture is further complicated by long-term drug therapy, with its potential for neurotoxicity, and psychosocial factors related to epilepsy, a chroni~cally disabling and stigmatizing illness.
FUTURE DIRECTIONS
While the relationship between epilepsy and schizophrenia-like psychosis has in itself been an intriguing clinical issue, it carries within it the potential to provide understanding of the pathogenesis of the schizophrenias in general. The newer epidemiological studies have been more sophisticated, but more work remains to be done. For example, the question of whether schizophrenia-like psychosis is specific to temporal lobe epilepsy should be further investigated by using extratemporal partial epilepsy and generalized epilepsy patients as comparison subjects. The determinants of the interval between the onset of epilepsy and that of psychosis should be studied in a longitudinal investigation of temporal lobe epilepsy patients beginning in the first or early second decade of life. It is important that brief psychoses be distinguished from the chronic psychoses in such investigations. Longitudinal studies are also necessary to determine the factors that lead to chronicity of psychosis in patients with repeated postictal and interictal psychoses. Chronic interictal psychoses studied longitudinally will help determine how seizures modulate the expression of psychotic symptoms. The boundary between postictal and brief interictal psychoses is poorly defined, and a comparison of the clinical features of these subgroups, as currently distinguished, as well as their ictal, EEG, and neuroimaging correlates, will be instructive. Methodologically superior family-genetic studies of schizophrenia-like psychosis will help answer the question of whether the epilepsy patients who become psychotic are so predisposed genetically.
Newer neurophysiological and neuroimaging techniques have not yet been sufficiently applied to the psychoses of epilepsy. Magnetoencephalography, with its potential to detect deep limbic discharges noninvasively, may help answer the question of whether prolonged psychosis can be produced by continuous subictal activity without abnormalities on scalp EEG. Because both anticonvulsants and antipsychotics may confound such studies, drug-free and preferably drug-naive subjects should be studied. Magnetic source imaging is also an excellent technique with which to examine schizophrenia-like psychosis for anomalous cerebral lateralization in view of the intriguing but uncertain literature on laterality of epilepsy and schizophrenia-like psychosis. The application of MRI, with its ability to image mesial sclerosis, heterotopias, and other developmental abnormalities and to perform volumetric assessments, can address a number of questions: Does mesial temporal sclerosis underlie schizophrenia-like psychosis in epilepsy? Do the patients who develop schizophrenia-like psychosis also have abnormalities of the temporal and/or frontal neocortices? Do they have heterotopias, abnormal gyral patterns, or cortical dysgenesis? Are large ventricles and low volume of the cortex, thalamus, cerebellum, etc., commonly reported in schizophrenia, also present in the chronic schizophrenia-like psychosis of epilepsy? Functional imaging studies, using PET, SPECT, or functional MRI, should examine the hypothesis of hypofrontality in schizophrenia-like psychosis if a final common pathway for the psychoses is hypothesized. PET and SPECT should be applied to the study of neurotransmitter function in schizophrenia-like psychosis, in particular to examine post- and presynaptic dopamine, serotonin, and glutamate functions.
Patients who received temporal lobe surgery and had a previous history of psychosis, or developed psychosis subsequently, remain an excellent resource for neuropathological studies. Nonpsychotic patients should be used for comparison, and surgically excised tissue should be examined for cell disarray, low numbers of certain cells (e.g., neurons containing dinucleotide phosphate diaphorase), small neuronal size, and abnormal cell migration. Newer techniques, such as immunocytochemistry and in situ hybridization for gene expression of messenger RNA, when applied to surgically excised tissue may help examine the dopamine hyperfunction, glutamate hypofunction, and other neurotransmitter hypotheses of schizophrenia in subjects with schizophrenia-like psychosis. Several techniques now available to identify candidate genes for cortical development could be applied to tissue from patients with schizophrenia-like psychosis. Finally, since animal models of epilepsy are well established, they provide a useful entry point to the development of suitable models for schizophrenia or psychotic disturbance.
 |
 |
Received Feb. 19, 1997; revision received July 23, 1997; accepted July 31, 1997. From the Neuropsychiatric Institute, The Prince Henry Hospital and School of Psychiatry, University of New South Wales, Sydney, Australia. Address reprint requests to Dr. Sachdev, Neuropsychiatric Institute, The Prince Henry Hospital, Little Bay, New South Wales 2036, Australia. The author thanks Drs. Michael Trimble and Janice Stevens for discussions, Prof. Gordon Parker for reading the manuscript, and Ms. Wanda Schinke and Ms. Helen Smartt for secretarial assistance.
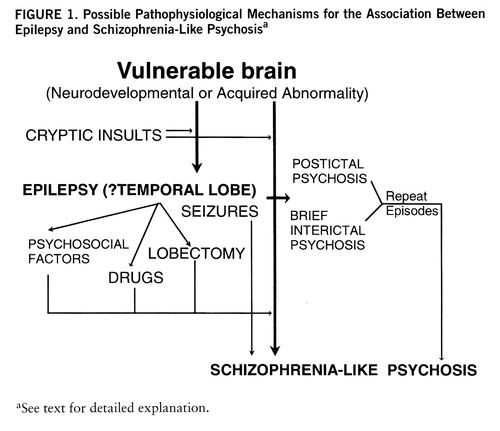
FIGURE 1. Possible Pathophysiological Mechanisms for the Association Between Epilepsy and Schizophrenia-Like Psychosisa
aSee text for detailed explanation.
1. Trimble M: The Psychoses of Epilepsy. New York, Raven Press, 1991Google Scholar
2. Mace CJ: Epilepsy and schizophrenia. Br J Psychiatry 1993; 163:439–445Crossref, Medline, Google Scholar
3. Mendez MF, Grau R, Doss RC, Taylor JL: Schizophrenia in epilepsy: seizure and psychosis variables. Neurology 1993; 43:1073–1077Google Scholar
4. Stevens JR: Clozapine: the yin and yang of seizures and psychosis. Biol Psychiatry 1995; 37:425–426Crossref, Medline, Google Scholar
5. Trimble MR, Ring HA, Schmitz B: Neuropsychiatric aspects of epilepsy, in Neuropsychiatry: A Comprehensive Textbook. Edited by Fogel B, Schiffer R, Rao S. Baltimore, Williams & Wil~kins, 1996, pp 771–803Google Scholar
6. Gastaut H, Gastaut JL, Goncalves e Silva GE, Fernandez-San~chez GE: Relative frequency of different types of epilepsy: a study employing the classification of the International League Against Epilepsy. Epilepsia 1975; 16:457–461Crossref, Medline, Google Scholar
7. Cummings JL: Frontal-subcortical circuits and human behavior. Arch Neurol 1993; 50:873–880Crossref, Medline, Google Scholar
8. Engel J Jr, Caldecott-Hazard S, Bandler R: Neurobiology of behavior: anatomic and physiologic implications related to epilepsy. Epilepsia 1986; 27(suppl 2):S3–S13Google Scholar
9. Dichter MA: Cellular mechanisms of epilepsy and potential new treatment strategies. Epilepsia 1989; 30(suppl 1):S3–S12Google Scholar
10. Slater E, Beard AW, Glithero E: The schizophrenia-like psychoses of epilepsy, i–v. Br J Psychiatry 1963; 109:95–150Crossref, Medline, Google Scholar
11. von Meduna L: über experimentelle Campherepilepsie. Archiv für Psychiatrie 1934; 102:333–339Crossref, Google Scholar
12. Lee SI: Nonconvulsive status epilepticus. Arch Neurol 1985; 42:778–781Crossref, Medline, Google Scholar
13. Scholtes FB, Renier WO, Meinardi H: Non-convulsive status epilepticus: causes, treatment, and outcome in 65 patients. J Neurol Neurosurg Psychiatry 1996; 61:93–95Crossref, Medline, Google Scholar
14. Engel J Jr, Bandler R, Griffith NC, Caldecott-Hazard S: Neurobiological evidence for epilepsy-induced interictal disturbances, in Neurobehavioral Problems in Epilepsy: Advances in Neurology, vol 55. Edited by Smith D, Treiman D, Trimble M. New York, Raven Press, 1991, pp 97–111Google Scholar
15. Devinsky O, Kelly K, Porter RJ, Theodore WH: Clinical and electroencephalographic features of simple partial seizures. Neurology 1988; 43:1347–1352Google Scholar
16. Williamson PD, Spencer SS: Clinical and EEG features of complex partial seizures of extratemporal origin. Epilepsia 1986; 27(suppl 2):S46–S63Google Scholar
17. Gloor P: Neurobiological substrates of ictal behavioral changes, in Neurobehavioral Problems in Epilepsy: Advances in Neurology, vol 55. Edited by Smith D, Treiman D, Trimble M. New York, Raven Press, 1991, pp 1–34Google Scholar
18. Kendrick JF, Gibbs FA: Origin, spread and neurosurgical treatment of the psychomotor type of seizure discharge. J Neurosurg 1957; 14:270–284Crossref, Medline, Google Scholar
19. Sem-Jacobsen CW: Depth electrographic observations on psychotic patients: a system related to emotional behaviour. Acta Psychiatr Scand 1959; 34:412–416Crossref, Google Scholar
20. Heath RG: Common clinical characteristics of epilepsy and schizophrenia: clinical observation and depth electrode studies. Am J Psychiatry 1962; 118:1013–1026Google Scholar
21. Fenwick P: The use of magnetocencephalography in neurology, in Advances in Neurology, vol 54: Magnetoencephalography. Edited by Sato S. New York, Raven Press, 1990, pp 271–282Google Scholar
22. Spencer SS, Spencer DD, Williamson PD, Mattson RH: The localizing value of depth electroencephalography in 32 patients with refractory epilepsy. Ann Neurol 1982; 12:248–253Crossref, Medline, Google Scholar
23. Logsdail SJ, Toone BK: Postictal psychosis: a clinical and phenomenological description. Br J Psychiatry 1988; 152:246–252Crossref, Medline, Google Scholar
24. So NK, Savard G, Andermann F, Olivier A, Quesney LF: Acute postictal psychosis: a stereo EEG study. Epilepsia 1990; 31:188–193Crossref, Medline, Google Scholar
25. Savard G, Andermann F, Olivier A, Remillard GM: Postictal psychosis after partial complex seizures: a multiple case study. Epilepsia 1991; 32:225–231Crossref, Medline, Google Scholar
26. Baumgartner C, Podreka I, Benda N, Olbrich A, Serles W, Novak K, Lurger S, Lindinger G: Postictal psychosis: a SPECT study (abstract). Epilepsia 1995; 36(suppl 3):S218Google Scholar
27. Kanner AM, Stagno S, Kotagal P, Morris HH: Postictal psychiatric events during prolonged video-electroencephalographic monitoring studies. Arch Neurol 1996; 53:258–263Crossref, Medline, Google Scholar
28. Kanemoto K, Takenchi J, Kawasaki J, Kawai I: Characteristics of temporal lobe epilepsy with mesial temporal sclerosis, with special reference to psychotic episodes. Neurology 1996; 47:1199–1203Google Scholar
29. Kanemoto K, Kawasaki J, Kawai I: Postictal psychosis: a comparison with acute interictal and chronic psychoses. Epilepsia 1996; 37:551–556Crossref, Medline, Google Scholar
30. Lancman ME, Craven WJ, Asconape JJ, Penry JK: Clinical management of recurrent postictal psychosis. J Epilepsy 1994; 7:47–51Crossref, Google Scholar
31. Umbricht D, Degreef G, Barr WB, Lieberman JA, Pollack S, Schaul N: Postictal and chronic psychoses in patients with temporal lobe epilepsy. Am J Psychiatry 1995; 152:224–231Link, Google Scholar
32. Ring HA, Trimble MR, Costa DC, Moriarty J, Verhoeff NP, Ell PJ: Striatal dopamine receptor binding in epileptic psychosis. Biol Psychiatry 1994; 35:375–380Crossref, Medline, Google Scholar
33. Meldrum BS: Neurochemical substrates of ictal behavior, in Neurobehavioral Problems in Epilepsy: Advances in Neurology, vol 55. Edited by Smith D, Treiman D, Trimble M. New York, Raven Press, 1991, pp 35–45Google Scholar
34. Reynolds EH: Interictal psychiatric disorders: neurochemical aspects. Ibid, pp 47–58Google Scholar
35. Tellenbach H: Epilepsie als Anfallsleiden und als Psychose: über alternative Psychosen paranoider PrÄgung bei “forcierter Normalisierung” (Landolt) des Elektroencephalogramms Epileptischer. Nervenarzt 1965; 36:190–202Medline, Google Scholar
36. Ramani V, Gumnit RJ: Intensive monitoring of interictal psychosis in epilepsy. Ann Neurol 1982; 11:613–622Crossref, Medline, Google Scholar
37. Wolf P: The clinical syndromes of forced normalization. Jpn J Psychiatry Neurol 1984; 38:187–192Google Scholar
38. Pakalnis A, Drake ME, John K, Kellum JB: Forced normalization: acute psychosis after seizure control in 7 patients. Arch Neurol 1987; 44:289–292Crossref, Medline, Google Scholar
39. Wolf P: Acute behavioral symptomatology at disappearance of epileptiform EEG abnormality: paradoxical or “forced” normalization, in Neurobehavioral Problems in Epilepsy: Advances in Neurology, vol 55. Edited by Smith D, Treiman D, Trimble M. New York, Raven Press, 1991, pp 127–142Google Scholar
40. Landolt H: Some clinical electroencephalographical correlations in epileptic psychoses (twilight states). Electroencephalogr Clin Neurophysiol 1953; 5:121Google Scholar
41. Kristensen O, Sindrup HH: Psychomotor epilepsy and psychosis. Acta Neurol Scand 1987; 57:361–379Crossref, Google Scholar
42. Schmitz B, Wolf P: Psychosis in epilepsy: frequency and risk factors. J Epilepsy 1995; 8:295–305Crossref, Google Scholar
43. Gibbs FA: Ictal and non-ictal psychiatric disorders in temporal lobe epilepsy. J Nerv Ment Dis 1951; 113:522–528Medline, Google Scholar
44. Landolt H: Serial electroencephalographic investigations during psychotic episodes in epileptic patients and during schizophrenic attacks, in Lectures on Epilepsy. Edited by Lorentz de Haas AM. Amsterdam, Elsevier, 1958, pp 91–133Google Scholar
45. Sander JWAS, Hart YM, Trimble MR, Shorvon SD: Vigabatrin and psychosis. J Neurol Neurosurg Psychiatry 1991; 54:435–439Crossref, Medline, Google Scholar
46. Chapman AG, Meldrum BS: Excitatory amino acid antagonists and epilepsy. Biochem Soc Trans 1993; 21:106–110Crossref, Medline, Google Scholar
47. Itil TM, Soldatos C: Epileptogenic side effects of psychotropic drugs. JAMA 1980; 244:1460–1463Google Scholar
48. Gibbs FA, Gibbs EL: Atlas of Electroencephalography, vol II: Epilepsy. Cambridge, Mass, Addison-Wesley, 1952Google Scholar
49. Currie S, Heathfield KWG, Henson RA, Scott DF: Clinical course and prognosis of temporal lobe epilepsy. Brain 1971; 94:173–190Crossref, Medline, Google Scholar
50. Standage KF, Fenton GW: Psychiatric profiles of patients with epilepsy: a controlled investigation. Psychol Med 1975; 5:152–160Crossref, Medline, Google Scholar
51. Onuma T, Adachi N, Ishida S, Katou M, Uesugi S: Prevalence and annual incidence of psychoses in patients with epilepsy (abstract). Epilepsia 1995; 36(suppl 3):S218Google Scholar
52. Krohn W: A study of epilepsy in Northern Norway: its frequency and character. Acta Psychiatr Scand Suppl 1961; 150:215–225Crossref, Google Scholar
53. Gudmundsson G: Epilepsy in Iceland—a clinical and epidemiological investigation. Acta Neurol Scand Suppl 1966; 25:1–124Google Scholar
54. Kat W: über den Gegensatz Epilepsie-Schizophrenie und das kombinierte Vorkommen dieser Krankheiten (Antagonism between epilepsy and schizophrenia and simultaneous occurrence of these diseases). Psychiatr en Neurol Bl 1937; 41:733–745Google Scholar
55. Davison K, Bagley CR: Schizophrenia-like psychoses associated with organic disorder of the central nervous system: a review of the literature, in Current Problems in Neuropsychiatry: British Journal of Psychiatry Special Publication 4. Edited by Herrington RN. Ashford, Kent, England, Headley Brothers, 1969, pp 1–45Google Scholar
56. Betts TA: Epilepsy and the mental hospital, in Epilepsy and Psychiatry. Edited by Reynolds EH, Trimble MR. Edinburgh, Churchill Livingstone, 1981, pp 175–184Google Scholar
57. Lindsay J, Ounstead C, Richards P: Long term outcome in children with temporal lobe seizures, III: psychiatric aspects in childhood and adult life. Dev Med Child Neurol 1979; 21:630–636Crossref, Medline, Google Scholar
58. Stevens JR: Psychosis and the temporal lobe, in Neurobehavioral Problems in Epilepsy: Advances in Neurology, vol 55. Edited by Smith D, Treiman D, Trimble M. New York, Raven Press, 1991, pp 79–96Google Scholar
59. Shorvon SD: Epidemiology, classification, natural history, and genetics of epilepsy. Lancet 1990; 336:93–96Crossref, Medline, Google Scholar
60. Hauser WA, Annegess JF, Rocca WA: Descriptive epidemiology of epilepsy—contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc 1996; 71:576–586Crossref, Medline, Google Scholar
61. Bruens JH: Psychoses in epilepsy. Psychiatr Neurol Neurochir 1971; 74:174–192Google Scholar
62. Perez MM, Trimble MR: Epileptic psychosis—diagnostic comparison with process schizophrenia. Br J Psychiatry 1980; 137:245–249Crossref, Medline, Google Scholar
63. Toone BK, Garralda ME, Ron MA: The psychoses of epilepsy and the functional psychoses. Br J Psychiatry 1982; 141:256–261Crossref, Medline, Google Scholar
64. Small JG, Milstein V, Stevens JR: Are psychomotor epileptics different? Arch Neurol 1962; 7:187–194Google Scholar
65. Shukla GD, Srivastava ON, Katiyar BC, Joshi V, Mohan PK: Psychiatric manifestations of temporal lobe epilepsy: a controlled study. Br J Psychiatry 1979; 135:411–417Crossref, Medline, Google Scholar
66. Hermann BP, Dikmen S, Schwartz MS, Karnes WE: Interictal psychopathology in patients with ictal fear: a quantitative investigation. Neurology 1982; 32:7–11Crossref, Medline, Google Scholar
67. Stevens JR: Epilepsy and psychosis: neuropathological studies of six cases, in Aspects of Epilepsy and Psychiatry. Edited by Trimble MR, Bolwig TG. New York, John Wiley & Sons, 1986, pp 117–146Google Scholar
68. Bruton CJ, Stevens JR, Frith CD: Epilepsy, psychosis, and schizophrenia: clinical and neuropathologic correlations. Neurology 1994; 44:34–42Crossref, Medline, Google Scholar
69. Flor-Henry P: Psychosis and temporal lobe epilepsy. Epilepsia 1969; 10:363–395Crossref, Medline, Google Scholar
70. Sherwin I: Psychosis associated with epilepsy: significance of laterality of the epileptogenic lesion. J Neurol Neurosurg Psychiatry 1981; 44:83–85Crossref, Medline, Google Scholar
71. Jensen I, Larsen JK: Psychoses in drug-resistant temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 1979; 42:948–954Crossref, Medline, Google Scholar
72. Perez MM, Trimble MR, Murray NMF, Reider I: Epileptic psychosis: an evaluation of PSE profiles. Br J Psychiatry 1985; 146:155–163Crossref, Medline, Google Scholar
73. Conlon P, Trimble MR, Rogers D: A study of epileptic psychosis using magnetic resonance imaging. Br J Psychiatry 1990; 156:231–235Crossref, Medline, Google Scholar
74. Gallhofer B, Trimble MR, Frackowiak R, Gibbs J, Jones T: A study of cerebral blood flow and metabolism in epileptic psychosis using positron emission tomography and oxygen. J Neurol Neurosurg Psychiatry 1985; 48:201–206Crossref, Medline, Google Scholar
75. Marshall JE, Syed GMB, Fenwick PBC, Lishman WA: A pilot study of schizophrenia-like psychosis in epilepsy using single-photon emission computerised tomography. Br J Psychiatry 1993; 163:32–36Crossref, Medline, Google Scholar
76. Fujimoto T, Takano T, Takeuch K, Nakmura K, Asakura T, Akimoto H: Proton magnetic resonance spectroscopy of temporal lobe in epileptic psychosis and schizophrenia (abstract). Epilepsia 1995; 36(suppl 3):S174Google Scholar
77. Roberts GW, Done DJ, Bruton C, Crow TJ: A “mock up” of schizophrenia: temporal lobe epilepsy and schizophrenia-like psychosis. Biol Psychiatry 1990; 28:127–143Crossref, Medline, Google Scholar
78. Onuma T, Adachi N, Hisano T, Uesugi S:10-year follow-up study of epilepsy with psychosis. Jpn J Psychiatry Neurol 1991; 45:360–361Google Scholar
79. Taylor DC: Factors influencing the occurrence of schizophrenia-like psychosis in patients with temporal lobe epilepsy. Psychol Med 1975; 5:249–254Crossref, Medline, Google Scholar
80. Bailey P, Green JR, Amador L, Gibbs FA: Treatment of psychomotor states by temporal lobectomy: a report of progress. Res Publ Assoc Res Nerv Ment Dis 1953; 31:341–346Medline, Google Scholar
81. Taylor DC: Mental state and temporal lobe epilepsy: a correlative account of 100 patients treated surgically. Epilepsia 1972; 13:727–765Crossref, Medline, Google Scholar
82. Polkey CE: Effects of anterior temporal lobectomy apart from the relief of seizures: a study of 40 patients. J R Soc Med 1983; 76:354–358Crossref, Medline, Google Scholar
83. Stevens JR: Psychiatric consequences of temporal lobectomy for intractable seizures: a 20–30 year follow-up of 14 cases. Psychol Med 1990; 20:529–545Crossref, Medline, Google Scholar
84. Mace CJ, Trimble MR: Psychosis following temporal lobe surgery: a report of six cases. J Neurol Neurosurg Psychiatry 1991; 54:639–644Crossref, Medline, Google Scholar
85. Barr WB, Ashtari M, Bilder RM, Degreef G, Lieberman JA: Brain morphometric comparison of first-episode schizophrenia and temporal lobe epilepsy. Br J Psychiatry 1997; 170:515–519Crossref, Medline, Google Scholar
86. Reith J, Benkelfat C, Sherwin A, Yasuhara Y, Kuwabara H, Andermann F, Bachneff S, Cumming P, Diksic M, Dyve SE, Etienne P, Evans AC, Lal S, Shevell M, Savard G, Wong DF, Chouinard G, Gjedde A: Elevated dopa decarboxylase activity in living brain of patients with psychosis. Proc Natl Acad Sci USA 1994; 91:11651–11654Google Scholar
87. Sato M: Long-lasting hypersensitivity to methamphetamine following amygdaloid kindling in cats: the relationship between limbic epilepsy and the psychotic state. Biol Psychiatry 1983; 18:525–536Medline, Google Scholar
88. Pollock DC: Models for understanding the antagonism between seizures and psychosis. Prog Neuropsychopharmacol Biol Psychiatry 1987; 11:483–504Crossref, Medline, Google Scholar
89. Levine DN, Finklestein S: Delayed psychosis after right temporo-parietal stroke and seizures or trauma: relation to epilepsy. Neurology 1982; 32:267–273Crossref, Medline, Google Scholar
90. Sutula T, He XX, Cavazos J, Scott G: Synaptic reorganization in the hippocampus induced by abnormal functional activity. Science 1988; 239:1147–1150Google Scholar
91. Babb TL, Kupfer WR, Pretorius JK: Synaptic reorganization of mossy fibers into molecular layer in human epileptic fascia dentata. Abstracts of the Society for Neuroscience 1988; 88:351Google Scholar
92. Babb TL, Brown WJ: Neuronal, dendritic, and vascular profiles of human temporal lobe epilepsy correlated with cellular physiology in vivo. Adv Neurol 1986; 949–966Google Scholar
93. Veith G: Anatomische Studie über die Ammonshornsklerose im Epileptikergehirn. Deutsche Zeitschrift für Nervenheilkunde 1970; 293–314Google Scholar
94. Meencke HJ, Janz D: Neuropathological findings in primary generalized epilepsy: a study of eight cases. Epilepsia 1984; 25:8–21Crossref, Medline, Google Scholar
95. Houser CR: Granule cell dispersion in the dentate gyrus of humans with temporal lobe epilepsy. Brain Res 1990; 535:195–204Crossref, Medline, Google Scholar
96. Weinberger DR, Lipska BK: Cortical maldevelopment, anti-psychotic drugs, and schizophrenia: a search for common ground. Schizophr Res 1995; 16:87–110Crossref, Medline, Google Scholar
97. Scheibel AB, Kovelman JA: Disorientation of the hippocampal pyramidal cell and its processes in the schizophrenic patient. Biol Psychiatry 1981; 16:101–102Google Scholar
98. Akbarian S, Bunney JE Jr, Potkin SG, Wigal SB, Hagman JO, Sandman CA, Jones EG: Altered distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase cells in frontal lobe of schizophrenia implies disturbance of cortical development. Arch Gen Psychiatry 1993; 50:169–177Crossref, Medline, Google Scholar
99. Benes FM: Altered glutamatergic and GABAergic mechanisms in the cingulate cortex of the schizophrenic brain. Arch Gen Psychiatry 1995; 52:1015–1018Google Scholar



