Effects of Cholecystokinin Tetrapeptide on Respiratory Function in Healthy Volunteers
Abstract
OBJECTIVE: The authors evaluated respiratory response to cholecystokinin tetrapeptide (CCK-4) in healthy volunteers. METHOD: Subjects were randomly assigned to either a CCK-4 (N=15) or placebo (N=15) challenge under double-blind conditions. RESULTS: Dyspnea was reported by all of the subjects who received CCK-4 but only one subject who received placebo. CCK-4 caused a significant increase in tidal volume and minute ventilation but had no effect on breathing frequency. Placebo had no effect on any of the respiratory measures. CONCLUSIONS: These data indicate that the behavioral effects of CCK-4 are accompanied by changes in respiration in healthy volunteers. (Am J Psychiatry 1998; 155:280–282)
Evidence suggests that panic disorder may be associated with disturbances in central respiratory function. Respiratory changes, experienced as dyspneic sensations, have been documented during and between panic attacks and are considered as core symptoms of such attacks (1). Moreover, several studies (2, 3) have demonstrated that marked increases in ventilation are a major physiological concomitant of pharmacologically induced panic. Panic attacks provoked by placebo infusion are also accompanied by increases in ventilation (2). It is interesting that significantly greater changes in ventilation have been detected during placebo-induced than during lactate-induced panic. If we assume a close analogy between placebo-induced and clinical panic attacks, this finding corroborates reports that panic is indeed accompanied by marked ventilatory activation and provisionally suggests that respiratory changes associated with laboratory-induced panic are not simply an artifact of the pharmacologic agent employed.
Central cholecystokinin (CCK) receptor agonists, such as CCK tetrapeptide (CCK-4), are potent panicogenic agents with well-characterized cardiorespiratory actions. In humans, systemic CCK-4 induces dyspnea at a high frequency (4), and in anesthetized dogs it produces a rapid increase in ventilation (5). In view of the suspected link between panic and respiratory dyscontrol, it is conceivable that the panicogenic effects of CCK-4 might result, in part, from an action of CCK on respiratory physiology. Accordingly, in the present study we evaluated the effect of CCK-4 on respiratory measures in healthy subjects.
METHOD
Thirty subjects, 14 men and 16 women (mean age=26.43 years, SD=5.8), were studied. They had no current or prior history of psychiatric disorders and no family history of psychiatric illness. They were physically healthy and drug free at the time of the experiment. The protocol was approved by the Health Protection Branch of Health and Welfare Canada and St. Mary's Hospital Ethics Committee. All subjects provided written informed consent.
The subjects reported to the Psychopharmacology Unit at St. Mary's Hospital at 9:00 a.m. after an overnight fast. They were randomly assigned to either a CCK-4 (N=15) or placebo (N=15) challenge. CCK-4, consisting of an amino acid chain of Trp-Met-Asp-Phe-NH2, was prepared according to previous protocols (4). Thirty minutes before the challenge each subject was seated in a comfortable recliner chair. An intravenous catheter was placed in an antecubital vein, through which physiological saline was slowly dripped. A blood pressure cuff with a pulse monitor attached to an automatic sphygmomanometer was applied to the other arm. After the initial setup, the subject was instructed to breathe continuously through a mouthpiece that was attached to a pneumotachograph. The pressure drop across the pneumotachograph was measured by a differential pressure transducer and sampled at 50 Hz by an analog-digital converter and stored on a 486 computer. Data acquisition was performed with the LABDAT and ANADAT data analysis packages (RHT-InfoDat, Montreal).
After a 10-minute accommodation period, baseline data were collected and the subject was given a bolus injection of CCK-4 (50 µg) or placebo. Behavioral response to the challenge was assessed with the Panic Symptom Scale, consisting of 18 DSM-III-R panic symptoms that are rated on a 0–4 scale. The Panic Symptom Scale provided a score for the total number of symptoms reported (number of symptoms with scores >1) and a sum intensity score. Symptoms not appearing on the Panic Symptom Scale but spontaneously reported by subjects were also recorded and analyzed separately. The DSM-III-R criteria for a panic attack, including anxiety, fear, and apprehension of at least moderate intensity (a score of ≥2 on this item on the Panic Symptom Scale), were used to define a panic attack. Vital signs were measured at 20-second intervals for 2 minutes after the challenge. Respiration was measured throughout the experiment.
We conducted analyses of variance (ANOVAs) and chi-square tests (with Yates's correction) to evaluate demographic characteristics, baseline variables, and behavioral response to the challenge. The cardiovascular data were submitted to ANOVA by using the maximum change from baseline (maximum value after injection minus the value at time 0) as the dependent variable. The respiratory data were analyzed with repeated measures ANOVA by using the average values of tidal volume, breathing frequency, and minute ventilation obtained during the 2½-minute period immediately before the challenge and the 2½-minute period immediately after the challenge. A two-tailed significance level of p≤0.05 was used for all analyses.
RESULTS
The subjects in the CCK group did not differ in demographic characteristics from those in the placebo group. Nevertheless, despite random assignment, significant baseline differences between the CCK-4 and placebo groups were detected for systolic blood pressure (CCK-4: mean=127.33 mm Hg, SD=13.7; placebo: mean=115.27, SD=12.8) (F=6.22, df=1,28, p<0.05) and diastolic blood pressure (CCK-4: mean=75.80 mm Hg, SD=8.7; placebo: mean=69.20, SD=7.4) (F=4.97, df=1,28, p<0.05). Accordingly, the baseline scores were used as covariates in the analyses of blood pressure data. No other significant baseline differences between the CCK-4 and placebo groups were noted.
As expected, the differences between CCK-4 and placebo on the behavioral measures were highly significant; the subjects given CCK-4 reported a greater number of symptoms (CCK-4: mean=10.53, SD=2.1; placebo: mean=1.27, SD=1.6) (F=182.76, df=1,28, p<0.001) and a greater sum intensity of symptoms (CCK-4: mean=26.45, SD=7.9; placebo: mean=1.67, SD=3.0) (F=128.51, df=1,28, p<0.001). There was no difference between the CCK-4 and placebo groups in the number of spontaneously reported symptoms (CCK-4: mean=0.53, SD=0.8; placebo: mean=0.20, SD=0.4) (F=1.93, df=1,28, p=0.18). Nine (60.0%) of the subjects who received CCK-4 experienced panic attacks, in contrast to only one subject (6.7%) who received placebo (χ2=7.35, df=1, p<0.01). With respect to the cardiovascular measures, significant differences between CCK-4 and placebo were found for the maximum increase in heart rate (CCK-4: mean=29.07 bpm, SD=20.9; placebo: mean=5.53, SD=5.5) (F=17.76, df=1,28, p<0.001), systolic blood pressure (CCK-4: mean=10.93 mm Hg, SD=12.0; placebo: mean=1.13, SD=4.7) (F=8.61, df=1,27, p<0.01), and diastolic blood pressure (CCK-4: mean=5.87 mm Hg, SD=5.8; placebo: mean=0.73, SD=3.4) (F=10.04, df=1,27, p<0.01); the CCK-4 group showed greater increases over baseline in all three measures.
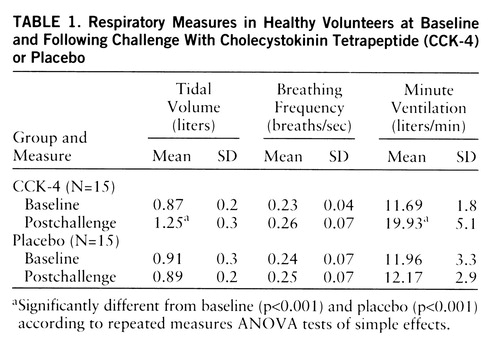
All of the subjects who received CCK-4 reported dyspnea, compared to only one subject who received placebo (χ2=22.63, df=1, p<0.001). The values for tidal volume, breathing frequency, and minute ventilation during the baseline and postchallenge periods appear in table 1. The ANOVA revealed no significant time effect (F=2.31, 1,28, p=0.14) or drug-by-time interaction (F=0.81, df=1,28, p=0.38) for breathing frequency. In contrast, significant time effects and drug-by-time interactions were detected for tidal volume (F=20.90 and 27.44, respectively, df=1,28, p<0.001) and minute ventilation (F=27.03 and 24.15, respectively, df=1,28, p<0.001). Within-group comparisons revealed baseline-to-postchallenge increases in the CCK-4 group for tidal volume (F=48.12, df=1,28, p<0.001) and for minute ventilation (F=51.13, df=1,28, p<0.001). Between-group comparisons revealed significant postchallenge differences between CCK-4 and placebo for tidal volume (F=15.03, df=1,28, p<0.001) and for minute ventilation (F=20.03, df=1,28, p<0.001). Although we did not identify significant differences between the CCK-4 panickers and nonpanickers in any of the respiratory measures, it is notable that the increase from baseline in minute ventilation was almost twice as great in the panickers as in the nonpanickers (panickers: mean=8.77 liters/minute, SD=5.5; nonpanickers: mean=4.95, SD=4.6).
DISCUSSION
The present study indicates that administration of CCK-4 to healthy volunteers provokes marked increases in ventilation. Consistent with ventilatory response to lactate infusion (2), inhalation of CO2 (3), and placebo infusion (2), this response was due to an increase in tidal volume rather than in breathing frequency. However, a previous study (5) showed that in anesthetized dogs the CCK-4-induced increase in ventilation was brought about principally by an increase in respiratory rate. In this study with dogs, tidal volume decreased despite an increase in respiratory drive simply because of the reduced breath period. The different pattern by which CCK-4 increases ventilation in conscious humans and in anesthetized dogs could be attributed to species differences or to the effect of anesthesia on the conscious control of breathing rate.
The specific mechanism of CCK-4's effect on respiration is still largely unknown. Nevertheless, studies of experimental animals suggest that CCK interacts with brainstem mechanisms in modulating respiratory function. Microiontophoretic application of CCK octapeptide into different regions of the nucleus tractus solitarius, a site of cardiopulmonary integration, decreases both neuronal firing and respiratory frequency in cats, effects that are reversed by administration of CCK-4 (6). Conceivably, the behavioral and physiological concomitants of panic induced by exogenous CCK-4 are mediated, in part, through stimulation of CCK receptors in brainstem regions.
In conclusion, our results suggest that CCK-4 is a potent respiratory stimulant. Additional research is needed to study respiratory response to CCK-4 in panic patients, to evaluate the effects of antipanic treatment on CCK-4-induced respiratory changes, and to evaluate the effects of CCK antagonists on respiratory changes induced by other panicogenic agents. This research might generate new insights into the role of CCK in respiratory dyscontrol in panic disorder in particular and in the neurobiology of panic disorder in general.
 |
Received Jan. 10, 1997; revision received June 9, 1997; accepted J uly 17, 1997. From the Department of Psychiatry and Meakins-Chris tie Laboratories,, McGill University, Montreal; the Psychopharmacology Unit, St. Mary's Hospital, Montreal; the Centre Hospitalier Paul Guiraude, Villejiuf, France; and the University of Nantes, Nantes, France. Address reprint requests to Dr. Bradwejn, Department of Psychiatry, University of Ottawa, 1145 Carling Ave., Ottawa, Ont. K1Z 7K4, Canada; [email protected] (e-mail). Supported by the St. Mary's Hospital Psychopharmacology Fund. Dr. Bates is a Fonds de la Recherche en Sante du Quebec scientist. The authors thank Lise Durand and Janice Johnson for assistance in this research.
1. Klein DF: False suffocation alarms, spontaneous panics, and related conditions: an integrative hypothesis. Arch Gen Psychiatry 1993; 50:306–317Crossref, Medline, Google Scholar
2. Goetz RR, Klein DF, Gully R, Kahn J, Liebowitz MR, Fyer AJ, Gorman JM: Panic attacks during placebo procedures in the laboratory: physiology and symptomatology. Arch Gen Psychiatry 1993; 50:280–285Crossref, Medline, Google Scholar
3. Gorman JM, Papp LA, Martinez J, Goetz, RR, Hollander E, Liebowitz MR, Jordan F: High-dose carbon dioxide challenge test in anxiety disorder patients. Biol Psychiatry 1990; 28:743–757Crossref, Medline, Google Scholar
4. Bradwejn J, Koszycki D, Shriqui C: Enhanced sensitivity to cholecystokinin tetrapeptide in panic disorder: clinical and behavioral findings. Arch Gen Psychiatry 1991; 48:603–610Crossref, Medline, Google Scholar
5. Bates JHT, Bradwejn J: Ventilatory response to cholecystokinin tetrapeptide in anesthetized dogs. NeuroReport 1995; 6:2513–2517Google Scholar
6. Denavit-Saubié M, Hurlé MA, Morin-Surun MP, Boutz AS, Champagnat J: The effects of cholecystokinin-8 in the nucleus tractus solitarius. Ann NY Acad Sci 1985; 448:375–384Crossref, Medline, Google Scholar



