Prospective Study of Tardive Dyskinesia in the Elderly: Rates and Risk Factors
Abstract
Objective:The purposes of this study were to investigate the rate (incidence) of tardive dyskinesia in elderly patients beginning treatment with antipsychotic medication and to identify risk factors for development of tardive dyskinesia in this age group. Method:A group of 261 neuroleptic-naive patients aged 55 or above were identified at the time they were starting antipsychotic drug treatment. This group is the complete study group; a preliminary report based on the first 160 patients was published previously. Patients were evaluated at baseline and followed up at 3-month intervals for periods ranging from 3 to 393 weeks. Assessments included abnormal involuntary movements, extrapyramidal signs, psychiatric symptoms, and medical and drug treatment histories.Results:The cumulative rates of tardive dyskinesia were 25%, 34%, and 53% after 1, 2, and 3 years of cumulative antipsychotic treatment. A greater risk of tardive dyskinesia was associated with history of ECT treatment, higher mean daily and cumulative antipsychotic doses, and presence of extrapyramidal signs early in treatment. Differences in tardive dyskinesia rates between diagnostic groups found in univariate analyses were attenuated when the authors controlled for these variables.Conclusions:Tardive dyskinesia rates for patients beginning treatment with conventional antipsychotics in their fifth decade or later are three to five times what has been found for younger patients, despite treatment with lower doses. Alternative treatments need to be investigated. Am J Psychiatry 1998; 155: 1521-1528
The earliest identified and most consistently reported finding in tardive dyskinesia research has been the increased vulnerability associated with aging. Greater prevalence, severity, and persistence of tardive dyskinesia with increasing age have been reported in many studies, and reviews have summarized these findings (1–4).
The methodological problems inherent in cross-sectional studies do not allow either a reliable estimate of the risk of tardive dyskinesia development for elderly patients for whom antipsychotic treatment is being considered or identification of factors that might affect this risk in the elderly. This is of considerable clinical importance given the widespread use of antipsychotic drugs to treat psychosis and behavioral disturbance in elderly patients; antipsychotics are prescribed for 25% to 45% of geriatric nursing home residents (5). Studies by others (6, 7) and by us (8) have focused on elderly patients examined for tardive dyskinesia at the time antipsychotics were first prescribed, or very early in treatment, and followed up for varying periods.
Yassa et al. (6) reported on 99 geriatric psychiatry inpatients examined at first antipsychotic treatment and reexamined up to 5 years later. Of the 99, 35 (35%) had tardive dyskinesia, defined as a rating of at least “mild” for one body area on the Abnormal Involuntary Movement Scale (AIMS) (9). When the more conservative Research Diagnosis of Tardive Dyskinesia criteria (10) were used, 30% had tardive dyskinesia. Prevalence rates by duration of antipsychotic drug treatment were as follows: 23% for those with 1–12 months, 50% for those with 13–24 months, 33% for those with 25–36 months, 57% for those with 37–48 months, and 46% for those with 49–60 months.
Patients who developed tardive dyskinesia were significantly younger and were hospitalized and treated for longer periods than those who did not. Diagnoses of major depressive disorder and alcoholic dementia were associated with high rates of tardive dyskinesia. The rate for men was higher than for women, significantly so when the Research Diagnosis of Tardive Dyskinesia criteria (10) were used. The data analyses did not explore the potential confounds between variables such as age, gender, diagnosis, and treatment.
Jeste et al. (7) prospectively examined 266 consecutively admitted Department of Veterans Affairs outpatients aged 45 and older who did not have abnormal movements at baseline. Most had been treated for less than 90 days at baseline and were treated continuously with low doses of antipsychotics.
The cumulative proportion of patients developing tardive dyskinesia by the end of 1 year was 26% (95% confidence interval=19% to 33%); by 2 years it was 52% (95% confidence interval=41% to 62%); and by 3 years it was 60% (95% confidence interval=48% to 72%). Multivariate analyses indicated that longer antipsychotic treatment before baseline, a larger amount of high-potency antipsychotics during follow-up, a history of alcohol abuse, a higher global AIMS score at baseline, and greater instrumental tremor measured during follow-up were significantly related to greater risk of tardive dyskinesia. The extent to which high potency, as opposed to high cumulative dose, was the influential variable was not clearly ascertained. Non-Caucasians and patients with diabetes had higher risks that were not statistically significant. Diagnosis, sex, and ratings of extrapyramidal signs were not related to tardive dyskinesia risk, although the instrumental tremor variable probably represents early extrapyramidal signs.
We reported preliminary results for the first 160 patients who participated in our prospective study of elderly patients followed from the beginning of their first course of antipsychotic treatment (8). We now present data for the complete study group of 261 patients.
METHOD
Subjects
Patients 55 years of age or older who were just beginning antipsychotic drug treatment were recruited from the geriatric services of Long Island Jewish-Hillside Medical Center, Glen Oaks, N.Y.; Parker Jewish Geriatric Center, New Hyde Park, N.Y.; and Beth Israel Medical Center, New York. The treating physician’s permission was obtained before the study was explained to patients and family members; oral agreement to participate was obtained. Written informed consent was obtained for special procedures (e.g., videotaping, computerized tomography [CT] scans), and written releases were obtained for records of prior treatment.
Patients showing abnormal involuntary movements with no history of antipsychotic drug treatment were excluded from the analyses, as were those with a previous history of antipsychotic treatment or with a neurological disorder (Huntington’s or Parkinson’s disease) that may have produced abnormal movements.
Assessments
The following were completed at study entry: medical records reviews and interviews with patients, relatives, and treatment staff to obtain demographic, medical, and psychiatric histories; semistructured interviews with ratings on the Mini-Mental State (11), Brief Psychiatric Rating Scale (BPRS), anchored version (12), and Global Assessment Scale (9); the Nurses’ Observation Scale for Inpatient Evaluation (NOSIE) (9); and a standardized examination for abnormal involuntary movements and extrapyramidal side effects with ratings on the modified Tardive Dyskinesia Rating Scale (13) and the Simpson-Angus Rating Scale (14). The Simpson-Angus scale was repeated weekly for the first 4 weeks, then quarterly; the Tardive Dyskinesia Rating Scale was repeated at 4 weeks and then quarterly. The NOSIE and medication history reviews were repeated every 3 months. The remaining scales were repeated every 6 to 12 months. Psychiatric and medical diagnoses were taken from the clinical records; DSM-III-R criteria were used at each study site. When more than one diagnosis was listed, the records were reviewed and a judgment was made to designate a primary diagnosis.
The Tardive Dyskinesia Rating Scale includes 28 items and a 6-point global score. In addition, the seven body-area items of the AIMS were rated. For the team of three trained raters, the interrater reliability (intraclass correlation coefficient) was 0.91 for the Tardive Dyskinesia Rating Scale global rating and 0.83 for the sum of the seven AIMS ratings.
Patients with a Tardive Dyskinesia Rating Scale global score of 1 (“questionable”) or higher were examined by a second rater, and in some cases a third, to confirm the severity level. A global rating of at least 2 (“mild”), by two independent examiners, was required to designate a “case” of tardive dyskinesia. For 97% of the tardive dyskinesia cases, the severity criteria of the Research Diagnosis of Tardive Dyskinesia (10) were also fulfilled; these require AIMS ratings of “mild” movements in two or more body areas or “moderate” or greater in one. Whenever possible, patients with presumptive tardive dyskinesia were videotaped and were given neurological examinations, clinical laboratory testing, and a noncontrast CT scan to identify possible false positive cases.
We did not participate in treatment decisions except to suggest antipsychotic discontinuation if tardive dyskinesia developed. Actually, the majority of patients, with tardive dyskinesia and without, were withdrawn from medication periodically by their clinicians.
Statistical Methods
We estimated cumulative rates of tardive dyskinesia by using life table analysis, and we used Cox proportional hazards regression to examine the effects of single and multiple potential risk factors. The time to development of tardive dyskinesia was calculated by starting from the day of the first antipsychotic dose regardless of whether the patient had been assessed for tardive dyskinesia at this time. Because treatment was often not continuous throughout follow-up, both analyses of single risk factors and multivariate regression analyses incorporated a time-varying covariate indicating whether individuals were or were not taking antipsychotic drugs. The proportional hazards assumption of Cox regression was tested by incorporating terms representing the interaction of the risk factor with time into the Cox models. Analyses that indicated changing hazard ratios over time were followed up with life table analyses to inspect the pattern of survival curves over time. Two-tailed tests of significance were used; 95% confidence intervals were computed for the hazard ratios.
We used survival analysis because of the different lengths of follow-up for the patients. The majority of patients did not develop abnormal movements during follow-up, leading to right-censored data, in which the time to event is unknown (having not yet occurred) but is known to be at least as long as the follow-up period. Survival analysis adjusts for right-censored data. Cox proportional hazards regression models were run by using the PHREG procedure of SAS (15). The LIFETEST procedure (16) was used for life table analysis.
Risk factors could be fixed at study entry (sex, race, diagnosis) or could vary with time (whether or not the patient was taking antipsychotic drugs, cumulative and mean daily antipsychotic drug dose). The cumulative drug dose was the total amount of drug in chlorpromazine equivalents received by a patient through the date of each tardive dyskinesia examination. The mean daily dose was calculated by dividing the cumulative drug dose for the period between ratings by the number of days between ratings.
Some patients had repeated assessments long after they stopped taking medication. A 100-day drug-free window was allowed wherein patients were considered still at risk for developing tardive dyskinesia. If patients who stopped taking and remained without antipsychotic drugs did not develop tardive dyskinesia within 100 days, their data was right-censored at the end of the 100 days. Thus, any dyskinesias diagnosed more than 100 days after medication discontinuation would not contribute to the tardive dyskinesia rate.
RESULTS
Study Group
We recruited 353 patients. For the analyses of tardive dyskinesia rates and risk factors we included patients who had at least 7 days of treatment with antipsychotic drugs and were followed up for at least 4 weeks. From the original group, 76 patients failed to meet these criteria: 20 never started taking antipsychotics, 10 had less than 1 week of exposure, six died, 27 refused follow-up, five were excluded because information indicating substantial prior antipsychotic treatment was later obtained, and eight were excluded for medical and other reasons.
Abnormal movements were present in 20 patients at baseline. Five of these were withdrawn from the study because of prior antipsychotic treatment, as just noted. Of the remaining 15, seven were antipsychotic drug naive and eight had some exposure (1, 1, 3, 5, 6, 11, 23, and 24 days, respectively). Another patient showed abnormal movements after baseline but before starting medication. These 16 patients were excluded from the analyses.
Subject Characteristics
Table 1 shows the characteristics of the 261 patients included in the analyses. The length of follow-up ranged from 3 to 393 weeks (mean=114.7 weeks). Baseline BPRS ratings suggested that for most patients, antipsychotic treatment was instituted in response to psychotic symptoms, either with agitation (51%) or alone (31%). Agitation alone was present in 9% of the patients, and 9% were not rated as either psychotic or agitated at baseline.
The medical histories included the following conditions: cardiovascular disorder, N=168 (64%); connective tissue disease, N=79 (30%); endocrine disorder, N=57 (22%); gastrointestinal disorder, N=62 (24%); and cancer, N=34 (13%). Nearly all (94%) of the patients had at least one chronic medical condition.
Antipsychotic Treatment During Study
The treatment and outcomes of these patients were previously reported in detail (17). The clear drug of choice at all sites was haloperidol, prescribed for 68% of the patients (N=177); a low-potency drug, usually thioridazine, was prescribed for 11% (N=30). Although treatment tended to be intermittent and/or short-term, 76% of the patients were treated continuously for the first month (N=198). Of the 163 patients followed for 1 year, 31% were treated for 49 weeks or more in the first year (N=51), and 21% were treated for 2 months or less (N=35).
The medication dose was very low for most patients. The average daily dose in chlorpromazine equivalents was 80 mg (SD=156) at the start and was 50 mg or less (≤1 mg haloperidol) for 51% of the 83 patients who were taking medication at 1 year (N=42). During the study, 55 patients received anticholinergic medication, most for short periods and in low doses.
Dyskinesia Cases Identified During Follow-Up
Of the 261 patients, 60 developed dyskinesia during follow-up. Most cases (77%) were rated as “mild” at the time of diagnosis, and the severity of tardive dyskinesia was never rated more than “mild” for 28 (47%). A severity rating of “moderate” was given to 24 cases (40%) at some point during the follow-up, and eight patients (13%) had moderately severe movements during at least one examination. Orofacial movements predominated and were always present whenever any movements were observed; 22 patients (37%) had only orofacial movements throughout, and 38 patients (63%) had movements in other body areas during at least one examination. The movements persisted for at least 3 months in 77% of the cases (N=46) and for at least 6 months in 67% (N=40). There were only four cases of transient (withdrawal) dyskinesia. Nine patients were not followed for 3 months; thus, the persistence of their tardive dyskinesia is indeterminate. For the 46 patients with persistent tardive dyskinesia, 39 (85%) had movements throughout the entire remaining follow-up; this was more than 1 year for 30 patients and more than 2 years for 23. The majority (61%) of the patients with persistent dyskinesia had abnormal movements after discontinuing antipsychotic treatment.
Tardive Dyskinesia Rate
The cumulative rate (incidence) of tardive dyskinesia was 20% (95% confidence interval=14%–26%) after 1 year, 30% (95% confidence interval=22%–38%) after 2 years, and 42% (95% confidence interval=32%–53%) after 3 calendar years of follow-up. Figure 1 illustrates the cumulative proportion of cases occurring and of the original subjects remaining at risk for tardive dyskinesia over time. After approximately 1, 2, and 3 years of cumulative antipsychotic exposure, the cumulative rates of tardive dyskinesia were 25% (95% confidence interval=18%–32%), 34% (95% confidence interval=26%–43%), and 53% (95% confidence interval=41%–66%), respectively (figure 2). While our preliminary data (8) suggested a plateau after 1 year, the data on the full study group indicate that cases continued to develop after this.
Risk Factors
Patients were more likely to show tardive dyskinesia when examined while not taking medication than when they were taking medication. The hazard ratio estimating the time-varying effect of being without medication was 3.25 (95% confidence interval=1.92–5.49; χ2=19.40, df=1, p<0.0001). The analyses of risk factors all incorporated the time-varying covariate indicating medication status into the model, thus controlling for whether or not the patients were taking antipsychotic drugs.
Patient variables
Men and women did not differ significantly in tardive dyskinesia vulnerability, and age was also not a significant predictor of tardive dyskinesia risk in this group of elderly patients. Other variables unrelated to tardive dyskinesia risk included race, educational level, substance abuse and smoking histories, use of anticholinergic medication, and number of medical problems noted. Patients with diabetes (defined as receiving oral hypoglycemic or insulin medication) were nonsignificantly more likely to develop tardive dyskinesia (hazard ratio=1.34, 95% confidence interval=0.68–2.66; χ2=0.70, df=1, p=0.40).
Preliminary life table analyses of tardive dyskinesia rates by diagnosis indicated that the rate was significantly higher for patients with multi-infarct dementia than for those with other organic mental syndrome diagnoses. Comparing the rates for patients with diagnoses grouped under “other axis I disorders” (table 1) revealed no significant differences between them. Nonetheless, given the literature suggesting that a diagnosis of mood disorder is a risk factor for tardive dyskinesia (18), we used this diagnosis as a separate category in subsequent analyses, which therefore compared the following four groups: 1) multi-infarct dementia (N=32); 2) Alzheimer’s dementia plus organic, delusional, and other organic mental syndromes (N=132); 3) major mood disorder (N=63); and 4) schizophrenia, schizoaffective disorder, and other axis I psychiatric disorders (N=34). The group with Alzheimer’s dementia plus other organic mental syndromes initially showed a lower rate of tardive dyskinesia (Wilcoxon χ2=9.36, df=3, p=0.03) than the other three diagnostic groups. This effect was lost after 2.5 calendar years of follow-up, although the small number of subjects remaining after this point dictates caution in interpretation (figure 3). When we used Cox regression analysis limited to the first 2 years of follow-up, the patients with major mood disorder (hazard ratio=2.23, 95% confidence interval=1.09–4.61; χ2=4.76, df=1, p=0.03), multi-infarct dementia (hazard ratio=2.18, 95% confidence interval=0.93–5.11; χ2=3.21, df=1, p=0.07), and schizophrenia and other axis I psychiatric disorders (hazard ratio=2.21, 95% confidence interval=0.95–5.18; χ2=3.36, df=1, p=0.07) had higher rates of tardive dyskinesia than the patients with Alzheimer’s dementia and other organic mental syndromes. The hazard ratios for the three groups with high rates were of similar magnitudes and did not differ statistically from each other. However, the mood disorder group was the only group with a significantly higher rate than the comparison group (Alzheimer’s dementia plus other organic mental syndromes).
Treatment variables
Higher risk of tardive dyskinesia was associated with higher mean daily and cumulative antipsychotic dosage early in treatment, but the effect diminished later on. Analyses limited to the first 2 years of follow-up revealed hazard ratios of 1.13 (95% confidence interval=1.03–1.23; χ2=7.39, df=1, p=0.007) per 10,000 mg of cumulative chlorpromazine-equivalent dose and 1.28 (95% confidence interval=1.09–1.50; χ2=8.98, df=1, p=0.003) per 100 mg of mean chlorpromazine-equivalent daily dose. History of treatment with ECT was a significant predictor of tardive dyskinesia (hazard ratio=6.97, 95% confidence interval=3.31–14.68; χ2=26.03, df=1, p=0.0001). Early extrapyramidal signs was a trend-level predictor but was stronger in analyses limited to the first 2 years of follow-up (hazard ratio=2.10, 95% confidence interval=1.15–3.83; χ2=5.81, df=1, p=0.02).
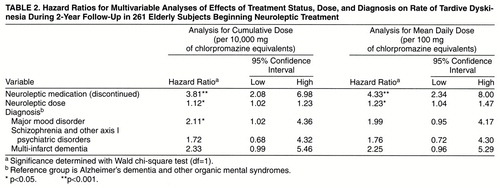
The variables significantly associated with development of tardive dyskinesia (time-varying antipsychotic status, antipsychotic dose, early extrapyramidal signs, history of ECT, and diagnosis) were included in two sets of multivariable Cox regression models to test for their independent effects. Since patients with diagnoses of organic mental syndrome received lower doses of medication than did those with other axis I disorders, the first set of analyses looked at the effects of antipsychotic status, diagnosis, and dose (expressed as cumulative and mean daily dosage). Table 2 lists hazard ratios, 95% confidence intervals, and significance levels for these variables. These analyses were limited to the first 2 years of follow-up because of the nonproportionality of hazard ratios over the full follow-up period and the small number of subjects at the later time points. The hazard ratios for antipsychotic status and dose were similar to those in the single-variable analyses already discussed and retained statistical significance. Patients diagnosed with mood disorders, schizophrenia and other axis I disorders, and multi-infarct dementia all had higher rates of tardive dyskinesia than the group with Alzheimer’s dementia and other organic syndromes. However, the only statistically significant hazard ratio was 2.11, for the mood disorder group in the analysis that used cumulative dose.
Table 3 presents hazard ratios, 95% confidence intervals, and significance levels for the second set of analyses, with early extrapyramidal signs and ECT added as covariates. Compared with the first set, the hazard ratio for major mood disorder was attenuated to 1.53 for the analysis using cumulative dose and 1.47 for the analysis using mean dose. There was little change in the hazard ratios for schizophrenia and other psychiatric disorders and for multi-infarct dementia. The cumulative-dose variable remained statistically significant, while the mean-dose variable failed to reach statistical significance. Early extrapyramidal signs and history of ECT continued to show strong and significant effects on the rate of tardive dyskinesia.
DISCUSSION
The tardive dyskinesia rates found for our complete study group are consistent with our preliminary findings with considerably narrowed confidence limits. Further, our rates are similar to those in the two other studies of older patients followed from the beginning of their antipsychotic treatment (6, 7). Thus, we are now confident that the rate of tardive dyskinesia for patients above age 50 who are beginning treatment with typical antipsychotics is three to five times what has been found for younger age groups (19, 20), despite the fact that the older patients are treated with much lower doses and the younger patients studied typically have long exposures to antipsychotics at baseline. Further, the movements are persistent and reach at least moderate levels of severity for the majority of the elderly patients affected.
In terms of risk factors, the results confirm our preliminary findings (8) that sex and increasing age are not related to the development of abnormal movements among geriatric patients. This may be due to the restricted age range and to the small number of men in the study group (a predominance of women is typical of geriatric groups). We have also confirmed our preliminary finding of an association between greater vulnerability to tardive dyskinesia and extrapyramidal signs early in antipsychotic treatment, and this is consistent with findings from our prospective study of younger, psychiatric patients (19) and with other reports for elderly groups (7).
Our preliminary analyses (8) indicated that diagnosis was important: patients with dementias and other organic mental syndromes were less vulnerable, while those with mood disorders and other axis I psychiatric diagnoses were more vulnerable to tardive dyskinesia. In our present analyses we used a further breakdown of the diagnostic groupings, a more thorough analysis of dose effects, and a more sophisticated statistical approach to the confound between diagnosis and dose. This revealed differences among the organic mental syndrome diagnoses: the risk of tardive dyskinesia among patients with multi-infarct dementia was similar to the rates for patients with mood disorder and those with other axis I psychiatric disorders, while patients with Alzheimer’s dementia and other organic mental syndromes showed a lower rate. There were strong effects of antipsychotic medication dose, and when these were controlled for, the differences between diagnostic groups were attenuated. We emphasize that the diagnoses were taken from the clinical charts, therefore potentially variable in quality, rendering our conclusions about diagnosis less secure.
Despite the small number of patients with a history of ECT, this remained a strong predictor of tardive dyskinesia vulnerability. Cole et al. (21) reported a similar finding, but most of the previous studies that examined ECT showed no effect on tardive dyskinesia risk (1, 22, 23); in one study there was an association between ECT and less vulnerability (24), and another study (25) indicated that ECT predicts a lower rate of tardive dyskinesia among schizophrenia patients and a higher rate among patients with mood disorder. Our finding may actually reflect a diagnostic effect. The mood disorder diagnosis may encompass a heterogeneous subset of this study group. Those treated with ECT could be a subgroup of patients with relatively severe or “true” mood disorders, more like those in younger age groups who have been found to be especially vulnerable to tardive dyskinesia (18).
ECT’s effects on the blood-brain barrier may also be relevant. Electrically induced seizures cause a transient disruption of the blood-brain barrier. The resulting enhanced permeability may produce higher brain concentrations of concurrently administered neuroleptics. There is evidence that peripheral levels of neuroleptics are transiently increased by ECT, but there are no data on CNS levels in humans (26). Further, some studies have shown enhanced dopamine-mediated behaviors, such as hypermotility and stereotypy, after ECT. Krueger and Sackheim (26) emphasized that this increase in dopaminergic tone is usually attributed to changes in D1 rather than D2 receptor function. Since it is clear that ECT has antipsychotic properties, it may not be surprising to see some effects in dopaminergic systems; however, the mechanism is far from clear, and the data regarding tardive dyskinesia are not adequate for drawing firm conclusions.
Questions may be raised about the validity of the diagnosis of drug-induced dyskinesia among geriatric patients, given their high rate of neuromedical conditions that themselves may be associated with abnormal movements resembling tardive dyskinesia and given the high rates of spontaneous dyskinesia in elderly patients reported in the literature. The 5% spontaneous dyskinesia rate we have observed is lower than the rates in some earlier surveys (27, 28). When movements emerge in the course of antipsychotic treatment among subjects free of movements before treatment, one may infer that the movements are not solely the result of a preexisting neuromedical condition. It is possible that short-term treatment by itself may not be sufficient to cause tardive dyskinesia but may contribute to the development of movements in neurologically predisposed individuals. In our study patients with diagnoses of organic mental syndromes other than multi-infarct dementia had a lower rate of tardive dyskinesia than did those with other axis I psychiatric diagnoses, and those with multi-infarct dementia had a nonsignificantly higher rate of tardive dyskinesia than patients with other organic mental syndrome diagnoses. This suggests that not all “organic” syndromes are potential predisposing factors for tardive dyskinesia.
The syndromes that are classified as organic mental disorders in DSM-III-R differ in terms of neurological systems affected, as well as etiology. Thus, it is not surprising that they may differ in their association with development of tardive dyskinesia. Multi-infarct dementia is associated with demonstrable structural brain abnormalities. It would be of interest to investigate whether the greater liability to tardive dyskinesia within the multi-infarct group is associated with specific structural lesions (such as those to the basal ganglia).
Since diabetes is a risk factor for cerebrovascular disease, including multi-infarct dementia, this is a possible mechanism for the connection between diabetes and abnormal movements that we (29) and others (30, 31) have reported. The fact that we failed to maintain the diabetes finding at a statistically significant level in this larger group may be related to our dependence on chart evidence rather than direct examination for presence of diabetes. Both diabetes and cerebrovascular insult are age-related factors that may underlie the heightened vulnerability of older people to tardive dyskinesia.
Our findings point up the need to investigate alternative treatments for patients in this age group. The new-generation antipsychotic agents are obvious considerations. Clozapine (32) and olanzapine (33) have been reported to have less tardive dyskinesia liability than the conventional drugs, but little is known about use of the new drugs for elderly subjects. This area of research clearly deserves attention.
Received June 9, 1997; revisions received Jan. 20 and May 14, 1998; accepted May 28, 1998. From the Department of Psychiatry, Hillside Hospital, Long Island Jewish Medical Center; and the Albert Einstein College of Medicine, Bronx, N.Y. Address reprint requests to Dr. Woerner, Psychiatry Research, Hillside Hospital, P.O. Box 38, Glen Oaks, NY 11004; [email protected] (e-mail). Supported in part by NIMH grants MH-40015 and MH-32369 and by NIMH Mental Health Clinical Research Center grant MH-41960 to Hillside Hospital.The authors thank Anthony Bossis, M.A., Melissa Smith, M.A., Linda Pestreich, and David Ryan for their participation in the data collection and Karen Blank, M.D., Jonathan Koblenzer, M.D., Paul Teusink, M.D., and Kenneth Kahaner, M.D., for assistance in patient recruitment.
 |
 |
 |
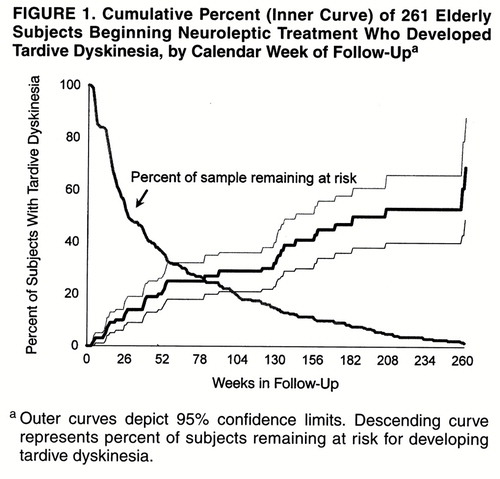
FIGURE 1. Cumulative Percent (Inner Curve) of 261 Elderly Subjects Beginning Neuroleptic Treatment Who Developed Tardive Dyskinesia, by Calendar Week of Follow-Upa
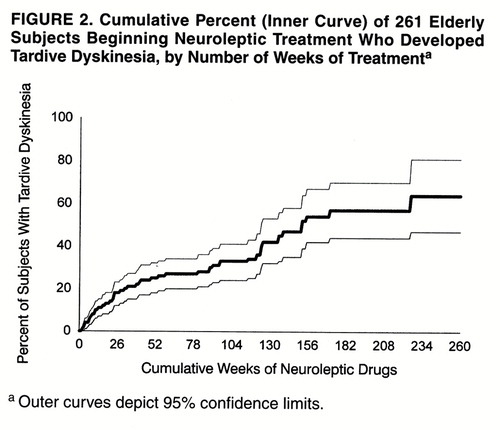
FIGURE 2. Cumulative Percent (Inner Curve) of 261 Elderly Subjects Beginning Neuroleptic Treatment Who Developed Tardive Dyskinesia, by Number of Weeks of Treatmenta
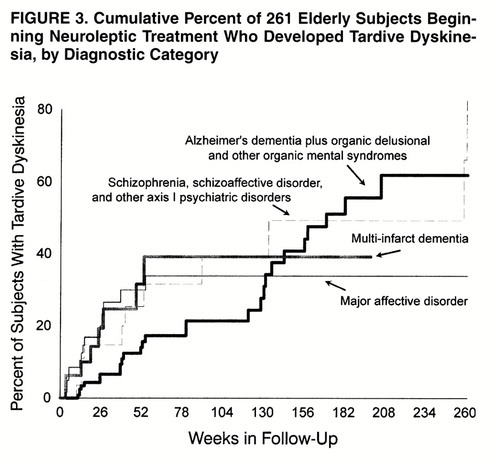
FIGURE 3. Cumulative Percent of 261 Elderly Subjects Beginning Neuroleptic Treatment Who Developed Tardive Dyskinesia, by Diagnostic Category
1. Kane JM, Smith J: Tardive dyskinesia: prevalence and risk factors—1959–1979. Arch Gen Psychiatry 1982; 39:473–481Crossref, Medline, Google Scholar
2. Smith JM, Baldessarini RJ: Changes in prevalence, severity, and recovery in tardive dyskinesia with age. Arch Gen Psychiatry 1980; 37:1368–1371Crossref, Medline, Google Scholar
3. Khot V, Wyatt RJ: Not all that moves is tardive dyskinesia. Am J Psychiatry 1991; 148:661–666Link, Google Scholar
4. Tardive Dyskinesia: A Task Force Report of the American Psychiatric Association. Washington, DC, APA, 1992Google Scholar
5. Harrington C, Tompkins C, Curtis M, Grant L: Psychotropic drug use in long-term care facilities: a review of the literature. Gerontologist 1992; 32:822–833Crossref, Medline, Google Scholar
6. Yassa R, Nastase C, Dupont D, Thibeau M: Tardive dyskinesia in elderly psychiatric patients: a 5-year study. Am J Psychiatry 1992; 149:1206–1211Link, Google Scholar
7. Jeste DV, Caligiuri MP, Paulsen JS, Heaton RK, Lacro JP, Harris MJ, Bailey A, Fell RL, McAdams LA: Risk of tardive dyskinesia in older patients: a prospective longitudinal study of 266 outpatients. Arch Gen Psychiatry 1995; 52:756–765Crossref, Medline, Google Scholar
8. Saltz BL, Woerner MG, Kane JM, Lieberman JA, Alvir JMJ, Bergmann KJ, Blank K, Koblenzer J, Kahaner K: Prospective study of tardive dyskinesia incidence in the elderly. JAMA 1991; 266:2402–2406Crossref, Medline, Google Scholar
9. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976Google Scholar
10. Schooler NR, Kane JM: Research diagnoses for tardive dyskinesia (letter). Arch Gen Psychiatry 1982; 39:486–487Medline, Google Scholar
11. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
12. Woerner MG, Mannuzza S, Kane JM: Anchoring the BPRS: an aid to improved reliability. Psychopharmacol Bull 1988; 24:112–117Medline, Google Scholar
13. Simpson GM, Lee JH, Zoubok B, Gardos G: A rating scale for tardive dyskinesia. Psychopharmacology (Berl) 1979; 64:171–179Crossref, Medline, Google Scholar
14. Simpson GM, Angus JWS: A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 1970; 212:11–19Crossref, Medline, Google Scholar
15. SAS/STAT Software: Changes and Enhancements, Release 6.10. Cary, NC, SAS Institute, 1994Google Scholar
16. SAS/STAT User"s Guide, version 6, 4th ed, vol 2. Cary, NC, SAS Institute, 1989Google Scholar
17. Woerner MG, Alvir JMJ, Kane JM, Saltz BL, Lieberman JA: Neuroleptic treatment of elderly patients. Psychopharmacol Bull 1995; 31:333–337Medline, Google Scholar
18. Gardos G, Casey D: Tardive Dyskinesia and Affective Disorders. Washington, DC, American Psychiatric Press, 1983Google Scholar
19. Kane JM, Woerner M, Lieberman J: Tardive dyskinesia: prevalence, incidence and risk factors. J Clin Psychopharmacol 1988; 8(suppl):52S–56SGoogle Scholar
20. Morgenstern H, Glazer W: Identifying risk factors of tardive dyskinesia among long-term outpatients maintained with neuroleptic medications: results of the Yale Tardive Dyskinesia Study. Arch Gen Psychiatry 1993; 50:723–733Crossref, Medline, Google Scholar
21. Cole JO, Gardos G, Boling LA, Marby D, Haskell D, Moore P: Early dyskinesia—vulnerability. Psychopharmacology (Berl) 1992; 107:503–510Crossref, Medline, Google Scholar
22. Rey JM, Hunt GE, Johnson GF: Assessment of tardive dyskinesia in psychiatric outpatients using a standardized rating scale. Aust NZ J Psychiatry 1981; 15:33–37Crossref, Medline, Google Scholar
23. Kok LP, Christopher YS: Tardive dyskinesia in schizophrenic outpatients. Ann Acad Med Singapore 1985; 14:87–90Medline, Google Scholar
24. Schwartz M, Silver H, Tal I, Sharf B: Tardive dyskinesia in northern Israel: preliminary study. Eur Neurol 1993; 33:262–266Crossref, Google Scholar
25. O’Hara P, Brugha TS, Lesage A, Wing J: New findings on tardive dyskinesia in a community sample. Psychol Med 1993; 23:453–465Crossref, Medline, Google Scholar
26. Krueger RB, Sackheim HA: Electroconvulsive therapy and schizophrenia, in Schizophrenia. Edited by Hirsch SR, Weinberger DR. Oxford, England, Blackwell Science, 1995, pp 305–545Google Scholar
27. Brandon S, McClelland HA, Protheroe C: A study of facial dyskinesia in a mental hospital population. Br J Psychiatry 1971; 118:171–184Crossref, Medline, Google Scholar
28. Delwaide PJ, Desseilles M: Spontaneous buccolingual-facial dyskinesia in the elderly. Acta Neurol Scand 1974; 56:256–262Crossref, Google Scholar
29. Woerner MG, Saltz BL, Kane JM, Lieberman JA, Alvir JMJ: Diabetes and development of tardive dyskinesia. Am J Psychiatry 1993; 150:966–968Link, Google Scholar
30. Ganzini L, Heintz RT, Hoffman WF, Casey DE: The prevalence of tardive dyskinesia in neuroleptic treated diabetics. Arch Gen Psychiatry 1991; 48:259–263Crossref, Medline, Google Scholar
31. Mukherjee S, Reddy R, Schnur DB: Diabetes mellitus and tardive dyskinesia. Biol Psychiatry 1991; 1:624–627Google Scholar
32. Kane JM, Woerner MG, Pollack S, Safferman AZ, Lieberman JA: Does clozapine cause tardive dyskinesia? J Clin Psychiatry 1993; 54:327–330Google Scholar
33. Tollefson GD, Beasley CM Jr, Tamura RN, Tran PV, Potvin JH: Blind, controlled, long-term study of the comparative incidence of treatment-emergent tardive dyskinesia with olanzapine or haloperidol. Am J Psychiatry 1997; 154:1248–1254Link, Google Scholar



