Clinical and Neurocognitive Aspects of Source Monitoring Errors in Schizophrenia
Abstract
OBJECTIVE: Source monitoring, an aspect of memory that involves judgments about the origin of information, has been found to be more prone to errors in schizophrenic subjects than in normal persons. To examine the precise nature of such errors and their relationship to clinical and neurocognitive variables, the authors compared schizophrenic and normal subjects. METHOD: Schizophrenic subjects who had been medication free for 1 week (N=26) and demographically matched normal subjects (N=21) performed a source monitoring task and were assessed on current psychiatric symptoms, IQ, and frontal lobe functioning. RESULTS: The schizophrenic subjects had normal recognition memory of target words (recognition hits) and a normal generation effect but made more errors than the comparison subjects in identifying the source of target words. Specifically, the schizophrenic subjects made more errors in remembering the source of new and self-generated items, and they tended to attribute items to an external source. In 11 retested subjects, these errors were stable and independent from medication status after a 2-year interval. Secondary analyses suggested that certain source monitoring errors may be associated with hostility and lower IQ. When the effect of IQ was controlled, correlations with frontal dysfunction were not significant. CONCLUSIONS: Schizophrenic subjects make significantly more source monitoring errors than normal subjects, but not because of problems with recognition memory hits or with the generation effect. This tendency may be trait like and may be related to hostility. Lower IQ in schizophrenia plays a partial role in these errors, but frontal dysfunction does not. (Am J Psychiatry 1997; 154:1530–1537)
Source monitoring is an aspect of memory that involves judgments about the origin, or source, of information (for a review, see reference 1). The source of information is the spatial, temporal, and contextual characteristics of an event as well as the sensory modalities through which it was perceived. The term “monitoring” implies that we retrieve and evaluate memories by using cognitive processes. The ability to discriminate among the sources of our memories allows us to remember whether an event actually happened to us, whether we simply imagined it, or whether we were told about it. In everyday life, source monitoring helps us to understand the origins of our own opinions and beliefs and thus contributes to our decisions and actions. In addition, autobiographical recollection—the ability to remember specific events in one's own life—depends on source memory attributions (1). Source memory and fact memory typically are independent functions (2).
Johnson et al. (1) proposed a model in which source monitoring is based on the characteristics of the memories themselves in combination with judgment processes. Greater cognitive elaboration occurs when we actively form memories of imagined events, as contrasted with the greater contextual and perceptual detail that occurs when we form memories for real events (3). Craik et al. (4) studied source amnesia (the forgetting of the source of a memory) in normal elderly subjects and found that it correlated with age, verbal fluency, and perseverative errors on the Wisconsin Card Sorting Test (5), but not with performance IQ or measures of fact recall. They concluded that the relation between mild degrees of frontal dysfunction and source amnesia is not secondary to general cognitive inefficiency. Johnson et al. (1) suggested that the roles of diencephalic and temporal regions in source monitoring are different from those of frontal regions of the brain. Diencephalic and temporal structures are likely involved in consolidating memory characteristics such as perceptual detail, while frontal regions are involved in discovering relations among events and in strategic retrieval.
Schizophrenic patients often describe a world in which internal stimuli are confused with real events. Frith (6) ascribed this to a disorder in central monitoring. Johnson (3) suggested that the perceptual aberrations and abnormal internal stimuli of schizophrenia wreak havoc with source monitoring and result in delusions. Harvey et al. (7, 8) demonstrated a relationship between source monitoring errors and disorganized thinking in schizophrenia. Bentall et al. (9) showed that, compared to nonhallucinating psychiatric subjects and normal subjects, hallucinating patients more often misattributed self-generated items to the experimenter.
The source monitoring errors found in schizophrenia consistently involve attribution of self-generated items to outside sources. However, there has been little examination of the precise nature of such errors or their relationship to clinical and neurocognitive variables. Likewise, it is not known whether the greater source monitoring errors in schizophrenia are simply due to the generalized cognitive deficit frequently observed in this disorder or whether, as in elderly normal subjects, they reflect a more specific underlying pathologic process.
In order to examine these issues, we studied 26 schizophrenic subjects who had been medication free for 1 week and 21 demographically matched normal comparison subjects. We analyzed their performance on a source monitoring task adapted from the experimental psychology literature. We also assessed subjects on current psychiatric symptoms, estimated IQ, and indices of frontal lobe functioning. Our questions were fivefold:
1. What specific types of source monitoring errors are made by schizophrenic subjects but not by normal comparison subjects?
2. What is the evidence that these errors represent a stable trait (versus state) phenomenon in schizophrenia?
3. What is the clinical significance of these errors? Are they associated with particular symptom clusters in schizophrenia? The literature alternately supports associations with perceptual aberrations (3, 9), delusional thinking (3, 6), thought disorder (7, 8), and by inference, the deficit syndrome (see references 4, 10, 11).
4. Is there a relationship between source monitoring errors and indices of frontal lobe dysfunction in schizophrenia, as has been demonstrated in normal elderly subjects (4) and in patients with frontal lobe lesions (10)?
5. Are source monitoring errors in schizophrenia simply a reflection of a generalized cognitive deficit, or do they represent a specific impairment?
METHOD
Subjects
All of the schizophrenic subjects (N=26) were clinically stable outpatients recruited from the Department of Psychiatry at the California Pacific Medical Center or the San Francisco Department of Veterans Affairs Medical Center. The normal comparison subjects (N=21) were recruited from hospital employees and from contacts in the community and were matched to the schizophrenic group on age, ethnicity, sex ratio, and mean parental education. The subjects were paid a nominal amount for their participation. After complete description of the study to the subjects, written informed consent was obtained.
The schizophrenic subjects were evaluated by using operationalized diagnostic criteria (DSM-III-R). The comparison subjects were screened to rule out any history of psychiatric illness or family history of schizophrenia. Additional exclusion criteria for all subjects were history of head trauma, neurological disorder, psychoactive substance abuse within the past 6 months (DSM-III-R criteria), and English as a second language.
The schizophrenic subjects participating in this experiment agreed, as part of the study protocol and with the support of their treatment team, to stop taking all psychiatric medications for 7 days before testing. The mean dose of neuroleptic for this group was 360 mg/day of chlorpromazine equivalents (range=100–750); low-dose anticholinergic medications had been used for 14 subjects and a short-acting benzodiazepine had been used for four subjects. No subject relapsed, experienced a clinically significant worsening of symptoms, or developed withdrawal dyskinesia during the brief washout period.
Source Monitoring Experiment
This experiment, adapted from a design by Mitchell and Hunt (12), used stimuli developed by Mitchell et al. (13).
Study phase. The subject is presented with a list of 40 sentences each containing a noun and a verb followed either by a target word (underlined) or by a fill-in-the-blank space. The sentences alternate: “The boy threw the ball” “The captain sailed the ____________” “The queen ruled the land” “The cat chased the ____________.”
The subject is asked to read each sentence aloud and to “make up a word and say it aloud” for each blank. The experimenter prints the subject's self-generated target words on a “test list.” No other instructions are given. The subject thus reads aloud 20 experimenter-generated target words and must self-generate and speak aloud 20 target words. The order of the sentence stimuli is varied among subjects.
Testing phase. The experimenter presents the test list to the subject approximately 2 hours later, after a number of other tasks (to be described). This list contains 40 word pairs from the original sentence stimuli, with target words that were generated by either the experimenter or the research subject (boy - ball, captain - ship, queen - land, cat - mouse). The test list also contains 20 new word pairs (recognition foils) containing words that are highly associated (e.g., bird - nest). The 60 word pairs on the test list are presented in pseudorandom order.
The research subject is told that the test list contains word pairs from the sentences that he or she read aloud in part 1 as well as some new word pairs. The subject is asked to determine, for each word pair, whether the target word is “brand new,” whether it is a word that he or she “made up and said aloud” when reading the original sentence, or whether it is a word that was underlined and that he or she simply “read aloud.”
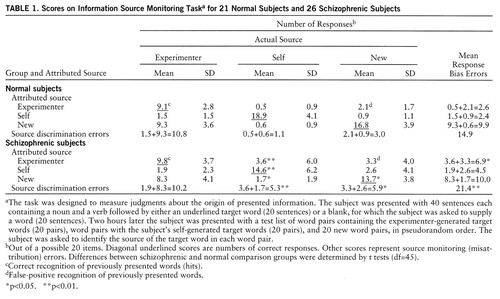
Overview of scoring. There are three possible actual sources for the target word in each word pair on the test list: it was previously presented to the subject and read aloud (experimenter generated), it was previously generated by the subject (self-generated), or it is a new target word. There are also three possible attributed sources for each target word (experimenter, self, new). This gives rise to nine (3×3) combinations of possible responses (table 1). Of these nine possible responses, six are misattribution errors—the subject says the target word is new when it is experimenter generated, the subject says the target word is new when it is self-generated, the subject says the target word is self-generated when it is new, etc. (table 1). There are thus two general classes of misattribution or source monitoring errors:
1. Response bias. This is the tendency to misattribute target words to a specific source, regardless of the actual source (sum of errors across each row in table 1). For example, a subject may show a response bias toward saying target words are experimenter generated when in fact two-thirds of them are either new or self-generated.
2. Impaired source discrimination. This is difficulty in identifying a specific source of target words (sum of errors down each column in table 1). For example, a subject may show greater difficulty in identifying the source of self-generated target words than in identifying the source of new target words.
Symptom Profile and Neurocognitive Assessment
All subjects were assessed with the Brief Psychiatric Rating Scale (BPRS) (14) and an executive-motor inventory (adapted from reference 15) on the day before the source monitoring experiment. The Wisconsin Card Sorting Test (5) and Shipley Institute of Living Scale (16) were administered on the day of the experiment.
The executive-motor inventory consisted of eight items from the Neurological Signs Inventory (15) that have localizing value for frontal dysfunction. The tests were fine-motor coordination, hand shapes, praxis to command, and five tests of prefrontal function: sequenced drawing, hand sequencing (fist-slap-cut), opposing action (tap twice when examiner taps once, and vice versa), alternating action (raise right or left hand in response to signals), go/no-go (perform or refrain from response to signals).
The Wisconsin Card Sorting Test (5), a measure of abstract problem solving and cognitive flexibility, has been found to be sensitive to frontal lobe dysfunction in many studies. The following Wisconsin Card Sorting Test indices were used: categories (number of rules discovered), perseverative error percentage (percentage of errors due to repeatedly following an incorrect rule), and set loss (number of failures to maintain correct sorting rule); scoring was performed according to the criteria of Heaton and Pendleton (17).
In a previous study (Poole et al., paper under review) in which we examined a group of 26 schizophrenic subjects and 18 normal comparison subjects that overlaps with the current group, a principal components analysis of the eight executive-motor scores and three Wisconsin Card Sorting Test scores revealed three factors that accounted for 67% of the total item variance: executive dysfunction (Wisconsin Card Sorting Test indices and sequential drawing), response disinhibition (failure to inhibit dominant motor responses on opposing action, alternating action, and go/no-go tasks), and motor dyscoordination (finger, hand, and foot coordination). These three executive-motor factors were used in the current study.
The Shipley Institute of Living Scale (16) is a brief, self-administered measure of general intellectual ability. Total score on this scale was used to estimate WAIS-R IQ, by means of age-normed conversions (18). In most studies, including those of psychiatric patients, the correlation (r) between the score on the Shipley Institute of Living Scale and the WAIS-R full-scale IQ is 0.73 to 0.85 (19).
General Procedure
One day before testing the BPRS and the executive-motor inventory were administered to the subjects. On the following day they received part 1 of the source monitoring experiment and then participated in a semantic priming experiment (20). After a rest period the Wisconsin Card Sorting Test and the Shipley Institute of Living Scale were administered, and the subjects then participated in part 2 of the source monitoring experiment. Independent research personnel administered each task and were blind to diagnosis and other test results.
For test-retest studies, 11 schizophrenic subjects were located an average of 2 years after initial participation and were invited to participate a second time in this project. They were not medication free during the second testing session. They were assessed with the Shipley Institute of Living Scale and participated in the same form of the experiment. Symptoms were assessed by using the Positive and Negative Symptoms Scale (21), but the executive-motor inventory and Wisconsin Card Sorting Test were not administered. The retest group was receiving a mean of 425 mg/day of chlorpromazine equivalents (range=50–700).
Data Analysis
A strong positive skew was present in the distribution of one-half of the measures of source monitoring error; these variables were normalized by either a logarithmic transformation (four measures) or an inverse transformation (two measures). Parametric tests of significance were then applied to the transformed variables. Internal reliability (Cronbach's alpha) and test-retest intraclass correlations were calculated for source monitoring error scores and for Shipley Institute of Living Scale estimated IQ. The test-retest reliability of comparable symptom ratings on the BPRS (test) and Positive and Negative Symptoms Scale (retest) was calculated by using Pearson correlations.
For inferential statistics, the method of protected F tests (Fisher's procedure, one-tailed) was used to control type I error rates. An initial multiple regression analysis was done as a test of overall significance. For each predictor found to contribute significantly in the overall test, univariate tests were performed. Where overall significance was not found, results of secondary univariate analyses are reported but interpreted cautiously.
The Pearson correlation matrix was calculated to characterize the relation of four specific source monitoring errors of interest to each other (correlation coefficients adjusted for item overlap), to symptom scores (BPRS), and to neurocognitive measures (three executive-motor factors and IQ) for the schizophrenic subjects. On the basis of prior studies of source monitoring, only unidirectional correlations were considered meaningful (i.e., source monitoring errors associated with worse symptoms and performance). Thus, one-tailed tests of significance were appropriate.
Finally, the relation of source monitoring errors to IQ was examined. Because IQ was strongly correlated with a diagnosis of schizophrenia in our group (F=16.3, df=1,45, p<0.001), the use of analysis of covariance was inappropriate (22). Instead, two steps were taken: 1) within the schizophrenic group, the partial correlations of BPRS scores and executive-motor factor scores with each source monitoring error of interest were reexamined, with the effect of IQ controlled for; and 2) by using a restricted subgroup of schizophrenic and normal subjects with IQs in the same range (range=95–115), group differences in source monitoring errors were reanalyzed.
RESULTS
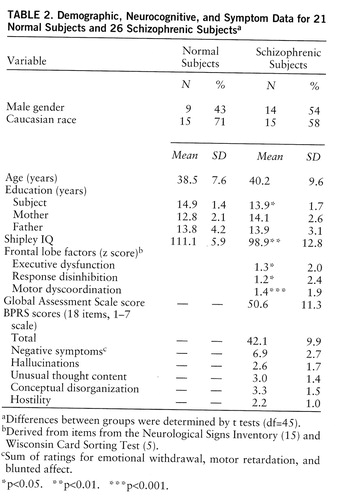
Table 2 presents demographic and cognitive characteristics for all of our subjects and the Global Assessment Scale (23) and BPRS scores for the schizophrenic group.
Table 1 presents the mean score for each possible type of source monitoring response by the normal comparison subjects and the schizophrenic subjects. The diagonal underlined scores are the numbers of correct responses. The other scores represent the six types of source monitoring (misattribution) errors.
The sums of errors across each row are the response bias errors (errors due to the tendency to attribute an item to a specific incorrect source, such as the experimenter). The sums of errors down each column are the source discrimination errors (due to difficulty in correctly attributing items coming from a specific source).
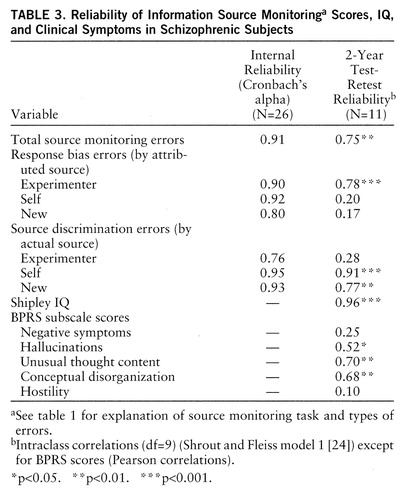
Internal reliability coefficients (Cronbach's alpha) for the source monitoring indices were calculated for the schizophrenic subjects and ranged from 0.76 to 0.95, which are adequate to excellent. Table 3 presents the internal reliability coefficients and the 2-year test-retest reliability data for a subgroup of 11 schizophrenic subjects. Three of the highest retest reliabilities were found for the response bias in which target items were attributed to the experimenter and for two types of source discrimination error. As will be discussed, these specific errors were characteristic of the schizophrenic group.
The clinical symptom ratings (BPRS at time 1 and related scores on the Positive and Negative Symptoms Scale at time 2 for retested subjects) showed significant test-retest correlations only for unusual thought content and conceptual disorganization.
A regression analysis was performed on the total subject group with total source monitoring errors as the dependent variable and the following three predictor variables: diagnosis, age, and education. There was a significant main effect of diagnosis only (F=7.4, df=1,45, p=0.009). Age, education, and all interaction effects were far from significant (p>0.4).
Group Comparisons
The schizophrenic subjects made significantly more total source monitoring errors than the comparison subjects (t=2.7, df=45, p=0.005); the mean z score difference was 1.0 (SD=1.3) relative to the comparison group (mean=0, SD=1). This moderately strong effect reflected the following between-group differences: 1) the schizophrenic subjects showed significantly more response bias in the form of attributing target words to the experimenter (t=2.1, df=45, p=0.02), a mean z score difference of 0.8 (SD=1.5); 2) the schizophrenic subjects made significantly more source discrimination errors when identifying the source of self-generated words (t=3.4, df=45, p=0.001), a mean z score difference of 1.3 (SD=1.6); and 3) the schizophrenic subjects made significantly more source discrimination errors when identifying the source of new target words (t=2.4, df=45, p=0.01), a mean z score difference of 1.3 (SD=2.5). As response bias errors overlap source discrimination errors, they were significantly correlated with both types of source discrimination errors (self-generated items: r=0.59, N=26, p=0.001; new items: r=0.71, N=26, p<0.001). However, the two types of source discrimination errors were not intercorrelated (r=0.23, N=26, p=0.13).
The schizophrenic subjects' performance on recognition of previously presented items (recognition hits) was identical (p>0.60) to that of the normal subjects. There also was no significant difference (p>0.15) in the number of recognition false positives (incorrectly reporting that a new item was previously presented by the experimenter). Both the schizophrenic and comparison subjects demonstrated a strong generation effect, i.e., more accurate recognition of the source of self-generated target words than experimenter-generated words (schizophrenic subjects: t=11.8, df=25, p<0.001; comparison subjects: t=12.8, df=20, p<0.001). When they misidentified the source of new items, both schizophrenic and normal subjects showed a significant tendency to identify the items as experimenter generated rather than self-generated (schizophrenic subjects: t=3.9, df=25, p<0.001; comparison subjects: t=3.5, df=20, p=0.001); this pattern has long been reported for normal subjects (25).
Relation to Clinical and Neurocognitive Aspects of Schizophrenia
Overall test of significance. A regression analysis was performed on the schizophrenic group with total source monitoring errors as the dependent variable and the following five predictor variables: IQ, BPRS total score, and the putative executive-motor factors of executive dysfunction, response disinhibition, and motor dyscoordination. The results of this test were not significant. Although significance was not attained in the multivariate test, secondary univariate analyses are reported here to facilitate future studies.
Univariate analyses. We performed secondary univariate analyses on total source monitoring errors and the two types of source discrimination errors that were more common in the schizophrenic group. Since these secondary analyses did not attain significance in the multivariate test, they must be interpreted with caution.
Total number of source monitoring errors was associated with BPRS hostility score (r=0.48, N=25, p=0.007), motor dyscoordination score (r=0.39, N=23, p=0.03), and lower IQ (r=–0.34, N=26, p=0.05). When the effect of IQ was partialed out, only the association with BPRS hostility score retained nominal significance (r=0.42, N=25, p=0.03).
Number of source discrimination errors related to self-generated target words was associated with BPRS hostility score (r=0.36, N=25, p=0.04), motor dyscoordination score (r=0.35, N=23, p=0.05), and lower IQ (r=–0.49, N=26, p=0.005). When the effect of IQ was partialed out, no significant correlations remained.
Number of source discrimination errors related to new target words was associated with higher BPRS hostility score (r=0.34, N=25, p=0.05). There was no association with the executive-motor factor scores or with IQ.
Relation to IQ
In the comparison subjects, all of whom had IQs in the normal range, IQ showed no significant correlation with total source monitoring errors or with either type of source discrimination error; there was a trend for lower IQ to be associated with the response bias of attributing items to the experimenter (r=–0.30, N=21, p=0.09). There was a significant positive correlation between source discrimination errors for new items and response disinhibition (r=0.44, N=20, p=0.03) and also executive dysfunction (r=0.41, N=20, p=0.04). Partialing out the effect of IQ did not change this observed relationship with response disinhibition (r=0.45, N=20, p=0.03), but it did slightly change the association with executive dysfunction (r=0.39, N=20, p=0.05).
Twenty schizophrenic subjects had IQs in a range comparable to that for our normal comparison subjects (range=95–115). These 20 schizophrenic subjects were not significantly different from the normal subjects on age or education, although IQ remained significantly different (schizophrenic subjects: mean=104.9, SD=6.1; normal subjects: mean=111.1, SD=5.6) (t=3.1, df=38, p=0.002). In this schizophrenic subgroup with normal IQs, the correlations between IQ and all source monitoring errors of interest were no longer significant (p>0.09 in all cases).
The subgroup of schizophrenic subjects with normal IQs still made significantly more errors than the comparison subjects in source discrimination regarding self-generated target items (t=2.4, df=39, p=0.02) but not new target items (t=1.7, df=39, p=0.10). They did not make significantly more total source monitoring errors (t=1.7, df=39, p=0.09) or response bias errors (t=0.9, df=39, p=0.45).
DISCUSSION
Summary of Findings
Specific types of source monitoring errors. Compared to demographically matched normal subjects, this group of moderately ill, clinically stable schizophrenic subjects had a strong bias toward saying that target items were generated by the experimenter, even when they were self-generated or new items. This bias contributed to poor source discrimination for both self-generated and new items, but these two types of source discrimination errors were independent from one another. This suggests that source monitoring difficulties in schizophrenia are due to two independent deficits: a failure to encode the source of self-generated items and a tendency to attribute new items to a previously presented source. Both of these deficits contribute to the cognitive bias toward attributing “puzzling” stimuli (self-generated items or new items) to an external source. The skewed distribution of these errors implies that only a subgroup of schizophrenic persons show these deficits.
Our data are consistent with those from several previous studies that have demonstrated source monitoring failures in schizophrenia (e.g., references 7–9, 26–28). Raye and Johnson (29) reported that the response bias error of attributing self-generated items to the experimenter is infrequent in normal subjects.
Trait-like aspects of source monitoring errors. In a subset of 11 retested schizophrenic subjects, we found that the pattern of source monitoring errors was stable over a 2-year period, despite a change from unmedicated to medicated status and fluctuations in the subjects' clinical symptoms. This suggests that impaired source monitoring in schizophrenia is a stable trait and not an artifact of symptoms or medication.
Harvey et al. (8) found that source monitoring was stable in schizophrenic inpatients over a 4-day interval and that at 8 months source monitoring variables did not differ significantly from baseline as a function of clinical status (26).
Clinical significance. Our schizophrenic subjects tended to attribute internally generated items to an external source, an error highly correlated with lower IQ (which also accounted for its association with hostility). Errors in identifying the source of new items were uncorrelated with IQ but may be associated with greater hostility. This suggests that hostility in schizophrenic patients may at times be related to difficulty in monitoring the source of information and to a tendency to experience information as coming from an external source. We propose that this bias is the cognitive foundation of projection.
Source monitoring errors were not associated with positive symptoms and were only weakly related to negative symptoms. Bentall et al. (9) found that schizophrenic patients with hallucinations more often misattributed self-generated items to the experimenter than did nonhallucinating schizophrenic subjects; Strauss et al. (30) concluded that hallucinations are related to problems in discriminating the source of information. Harvey et al. (8) found that errors in reality monitoring were associated with thought disorder, but in another study Harvey and colleagues (26) also highlighted the instability of positive symptom indices and their cognitive correlates within and across subject groups.
Neurocognitive findings. Although the findings from our secondary analyses within the schizophrenic group must be interpreted with caution (because of the absence of significance in the multivariate test), we found an association of executive-motor dysfunction with source monitoring errors that was mediated by lower IQ in the schizophrenic subjects. Lower IQ showed a significant relationship with source discrimination errors for self-generated items but not new items. In a subgroup of 20 schizophrenic subjects with IQs in the normal range, there were no longer any significant relationships between IQ and source monitoring errors, and yet this subgroup continued to show a significant difference in source discrimination errors from the normal subjects. Our data thus suggest that 1) the hypothesized influence of frontal dysfunction on source monitoring errors in schizophrenia is not verified independently of IQ, and 2) general cognitive inefficiency—although it makes an important contribution to source monitoring errors—is not the sole contributing factor.
To our knowledge, prior research has not addressed either IQ or frontal function in relation to source monitoring errors in schizophrenia. Craik et al. (4) found that source memory scores did not correlate with nonverbal intelligence in older adults but were associated with lower levels of education and achievement. Garety et al. (31) found that delusional schizophrenic patients with extreme reasoning biases had lower verbal intelligence than subjects without these extreme responses. Source memory is impaired in the elderly (4) and in patients with frontal lobe lesions (10), suggesting that intact frontal functions are critical for associating memories with the context in which they are learned. However, frontal dysfunction is probably only one of several factors that contribute to source monitoring errors. Dywan et al. (32) found that source monitoring errors in older adults were not related to performance on the Wisconsin Card Sorting Test but depended on various attentional control processes, not all of which were frontally based. Spencer and Raz (11), who also found no neuropsychological evidence of frontal lobe involvement in age-related impairment of source discrimination, proposed that an impaired ability to divide attention between target and context could result in the encoding of content at the expense of source.
Normal aspects of source monitoring in schizophrenia. Our schizophrenic subjects were not significantly different from normal subjects in identifying the source of previously presented target words (recognition memory hits) or in the generation effect (i.e., they made fewer errors in recognizing self-generated than experimenter-generated target words). Like the normal subjects, the schizophrenic subjects processed self-generated items differently from experimenter-presented items, and they had access to this difference during the act of remembering.
When they misidentified the source of new items, the schizophrenic patients, like the normal subjects, tended to identify the words as experimenter generated rather than self-generated. As Johnson et al. (25) noted, when an item is familiar but the memory trace does not include information about its source, people have a tendency to conclude that it was external in origin. However, schizophrenic subjects make many more of these response bias errors and combine them with a specific problem in remembering the source of self-generated items (an error unusual for normal subjects).
Methodological Issues
The relatively small number of subjects in this study and the mild to moderate clinical symptoms of the schizophrenic patients may have led to type II errors (e.g., weak or absent relationships of clinical symptoms and executive-motor measures to source monitoring in schizophrenia). Assessing a larger and more clinically impaired subject group with a more focused instrument than the BPRS would provide useful data. Detailed neurocognitive assessment, including a more extensive neuropsychological test battery, could provide crucial information, including the relation of source monitoring to attention, the ability to use context, and specific details of memory function. We cannot, at this time, make strong conclusions about the relevance of frontal functioning to source monitoring in the absence of a larger battery of tasks.
The lack of a psychiatric comparison group in this study prevents us from making any conclusions about the specificity of our findings to schizophrenia. In addition, the relatively narrow range of IQs in the normal comparison subjects does not allow us to say whether the relationship found between source monitoring errors and IQ in schizophrenia also is present in normal subjects.
Clinical Implications
This study demonstrates that schizophrenic subjects, unlike normal subjects, tend to attribute both new and self-generated information to an external source and that these errors are stable and independent from medication status after a 2-year interval for a subgroup of subjects. These errors may be related to hostility, and IQ plays a significant, although only partial role; frontal dysfunction showed no effect independent of IQ. What are the clinical implications of these findings?
First, patients with psychotic illness consistently attribute internally generated events (hallucinations and delusions) to an external source. Traditional models in psychiatry emphasize psychodynamic causes for this phenomenon. Using an experiment with affectively neutral stimuli, we have underscored its cognitive component and its relationship to hostility. Delusional thinking in patients is resistant to medication effects, an observation consistent with our finding that source monitoring errors are independent of medication status and may be a stable neurocognitive trait.
Second, although general cognitive inefficiency plays an important role in source monitoring, it does not account for all of the types of errors made by schizophrenic persons, and even patients with IQs in the normal range make significantly more source discrimination errors for self-generated items than do normal subjects. Furthermore, frontal dysfunction is not strongly or independently associated with source monitoring errors. This may account for the clinical observation that highly functioning or neurocognitively “intact” patients can have source discrimination problems (delusional ideation) in the absence of the severe stigmata of chronic psychosis (i.e., a deteriorating course, lower IQ, extreme thought disorder).
We propose that source monitoring problems represent a specific cognitive deficit associated with some forms of psychotic illness. While it is well known that the cognitive bias of schizophrenic and delusional patients is to focus on stimuli that are strong by normal standards and to neglect weaker stimuli (31, 33), this does not explain why schizophrenic patients make more source discrimination errors than normal subjects for self-generated items. The well-understood generation effect—which schizophrenic subjects demonstrate—indicates that self-generated items are processed as stronger stimuli (because of their multiple associated cognitive traces). Additionally, even for normal subjects, when internally generated responses are highly related to ongoing external stimuli and when they are highly controlled by external cues, these responses are difficult to discriminate from external events (25). In this experiment, internal and external events were separable enough to obtain a generation effect for both subject groups, and yet the schizophrenic subjects still made more source monitoring errors.
We hypothesize that, in acute phases of schizophrenia, patients experience a mixture of puzzling stimuli: abnormal sensory, cognitive, and affective information, often combined with emotionally or psychosocially demanding situations. Patients generate an explanation for the confusing and overwhelming experiences. Because of source monitoring difficulties, they attribute both the experiences themselves and the internally generated explanations to external sources, and the data, although originally self-generated, become indistinguishable from externally generated information.
The self in the paranoid-schizoid position is the self as object, not the self as creator and interpreter of one's thoughts, feelings, perceptions, and the like. . . . The patient . . . /does/ not experience himself as an active personal agent but, rather, as an object to whom life events occur. (34, pp. 48–49)
We are thus brought full circle to observations made a decade ago by object relations theorists and to contemplation of a bridge between cognitive and psychodynamic approaches to the puzzle of psychosis.
 |
 |
 |
Received Jan. 21, 1997; revision received May 27, 1997; accepted June 4, 1997. From the Psychiatry Outpatient Service, Department of Veterans Affairs Medical Center, San Francisco. Address reprint requests to Dr. Vinogradov, Psychiatry Service (116C), Department of Veterans Affairs Medical Center, 4150 Clement St., San Francisco, CA 94121; [email protected] (e-mail). Supported in part by a grant from the Scottish Rite Schizophrenia Research Program and by a Health Services Research and Development Ancillary Funding Award from the Department of Veterans Affairs to Dr. Vinogradov. The authors thank Leora Benioff, Ph.D., and Henry Skinner, B.A., for their help in screening, assessing, and testing subjects.
1. Johnson MK, Hashtroudi S, Lindsay DS: Source monitoring. Psychol Bull 1993; 114:3–28Crossref, Medline, Google Scholar
2. Shimamura AP, Squire LR: A neuropsychological study of fact memory and source amnesia. J Exp Psychol Learn Mem Cogn 1987; 13:464–473Crossref, Medline, Google Scholar
3. Johnson MK: Discriminating the origin of information, in Delusional Beliefs. Edited by Oltmanns TF, Maher BA. New York, John Wiley & Sons, 1988, pp 34–65Google Scholar
4. Craik FI, Morris LW, Morris RG, Loewen ER: Relations between source amnesia and frontal lobe functioning in older adults. Psychol Aging 1990; 5:148–151Crossref, Medline, Google Scholar
5. Berg EA: A simple objective treatment for measuring flexibility in thinking. J Gen Psychol 1948; 39:15–22Crossref, Medline, Google Scholar
6. Frith CD: The positive and negative symptoms of schizophrenia reflect impairments in the perception and initiation of action. Psychol Med 1987; 17:631–648Crossref, Medline, Google Scholar
7. Harvey PD: Reality monitoring in mania and schizophrenia: the association of thought disorder and performance. J Nerv Ment Dis 1985; 173:67–73Crossref, Medline, Google Scholar
8. Harvey PD, Earle-Boyer EA, Levinson JC: Cognitive deficits and thought disorder: a retest study. Schizophr Bull 1988; 14:57–66Crossref, Medline, Google Scholar
9. Bentall RP, Baker GA, Havers S: Reality monitoring and psychotic hallucinations. Br J Clin Psychol 1991; 30:213–222Crossref, Medline, Google Scholar
10. Janowsky JS, Shimamura AP, Squire LR: Source memory impairment in patients with frontal lobe lesions. Neuropsychologia 1989; 27:1043–1056Google Scholar
11. Spencer WD, Raz N: Memory for facts, source, and context: can frontal lobe dysfunction explain age-related differences? Psychol Aging 1994; 9:149–159Google Scholar
12. Mitchell DB, Hunt RR: How much “effort” should be devoted to memory? Mem Cogn 1989; 17:337–348Google Scholar
13. Mitchell DB, Hunt RR, Schmitt FA: The generation effect and reality monitoring: evidence from dementia and normal aging. J Gerontol 1986; 41:79–84Crossref, Medline, Google Scholar
14. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Crossref, Google Scholar
15. Merriam AE, Kay SR, Opler LA, Kushner SF, Van Praag HM: Neurological signs and the positive-negative dimension in schizophrenia. Biol Psychiatry 1990; 28:181–192Crossref, Medline, Google Scholar
16. Shipley WC: The Shipley Institute of Living Scale for measuring intellectual impairment, in Contributions Toward Medical Psychology, vol 2: Theory and Diagnostic Methods. Edited by Weider A. New York, Ronald Press, 1953, pp 751–756Google Scholar
17. Heaton RK, Pendleton MG: Use of neuropsychological tests to predict adult patients' everyday functioning. J Consult Clin Psychol 1981; 49:807–821Crossref, Medline, Google Scholar
18. Zachary RA, Paulson MJ, Gorsuch RL: Estimating WAIS IQ from the Shipley Institute of Living Scale using continuously adjusted age norms. J Clin Psychol 1985; 41:820–831Crossref, Medline, Google Scholar
19. Kaufman AS: Assessing Adolescent and Adult Intelligence. Needham Heights, Mass, Allyn & Bacon, 1990Google Scholar
20. Ober BA, Vinogradov S, Shenaut GK: Automatic and controlled semantic priming in schizophrenia. Neuropsychology (in press)Google Scholar
21. Kay SR, Sevy S: Pyramidical model of schizophrenia. Schizophr Bull 1990; 16:537–545Crossref, Medline, Google Scholar
22. Strauss ME, Allred LJ: Measurement of differential cognitive deficits after head injury, in Neurobehavioral Recovery From Head Injury. Edited by Levin HS, Grafman J, Eisenberg HM. New York, Oxford University Press, 1987, pp 88–107Google Scholar
23. Endicott J, Spitzer RL, Fleiss JL, Cohen J: The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 1976; 33:766–771Crossref, Medline, Google Scholar
24. Shrout PE, Fleiss JL: Intraclass correlations: uses in assessing rater reliability. Psychol Bull 1979; 86:420–428Crossref, Medline, Google Scholar
25. Johnson MK, Raye CL, Foley HJ, Foley MA: Cognitive operations and decision bias in reality monitoring. Am J Psychol 1981; 94:37–64Crossref, Google Scholar
26. Harvey PD, Docherty NM, Serper MR, Rasmussen M: Cognitive deficits and thought disorder, II: an 8-month followup study. Schizophr Bull 1990; 16:147–156Crossref, Medline, Google Scholar
27. Harvey PD, Serper MR: Linguistic and cognitive failures in schizophrenia: a multivariate analysis. J Nerv Ment Dis 1990; 178:487–493Crossref, Medline, Google Scholar
28. Huron C, Danion J-M, Giacomoni F, Grangé D, Robert P, Rizzo L: Impairment of recognition memory with, but not without, conscious recollection in schizophrenia. Am J Psychiatry 1995; 152:1737–1742Google Scholar
29. Raye CL, Johnson MK: Reality monitoring vs discriminating between external sources of memories. Bull Psychonomic Society 1980; 15:405–408Crossref, Google Scholar
30. Strauss ME, Buchanan RW, Hale J: Relations between attentional deficits and clinical symptoms in schizophrenic outpatients. Psychiatry Res 1993; 47:205–213Crossref, Medline, Google Scholar
31. Garety PA, Hemsley DR, Wessely S: Reasoning in deluded schizophrenic and paranoid patients: biases in performance on a probabilistic inference task. J Nerv Ment Dis 1991; 179:194–201Crossref, Medline, Google Scholar
32. Dywan J, Segalowitz SJ, Williamson L: Source monitoring during name recognition in older adults: psychometric and electrophysiological correlates. Psychol Aging 1994; 9:568–577Crossref, Medline, Google Scholar
33. Chapman LJ, Chapman JP: The genesis of delusions, in Delusional Beliefs. Edited by Oltmanns TF, Maher BA. New York, John Wiley & Sons, 1988, pp 167–183Google Scholar
34. Ogden TH: The Matrix of the Mind. Northvale, NJ, Jason Aronson, 1990Google Scholar



