Neuroleptic Malignant Syndrome
“Ms. A,” a 25-year-old woman with paranoid schizophrenia, presented with an acute psychotic episode after having stopped taking her medication. She reported auditory hallucinations and was noted to be disheveled and to have loud, pressured speech, disorganization of thought processes, and persecutory delusions. Over a 10-hour period in the emergency department, she received two intramuscular injections of haloperidol (5 mg each) and required intermittent physical restraint for safety. Her prior regimen of 10 mg/day of oral haloperidol and 1 mg/day of benztropine was resumed. The following morning, Ms. A was noted to be diaphoretic and in moderate distress. Her heart rate was 140 bpm, blood pressure 145/92 mmHg, respiratory rate 26 breaths per minute, and temperature 104.5°F (rectal). Physical examination demonstrated generalized rigidity and tremulousness in all extremities, and her mental status was consistent with delirium. Laboratory studies were remarkable for a white blood cell count of 15,000 cells/ml and a creatine kinase level of 45,050 IU. Levels of serum transaminases were elevated, but levels of electrolytes, blood urea nitrogen, and creatinine were all within normal limits, and a urine toxicology screen was negative. Results of a CSF examination were normal, and cultures of blood and urine were negative. A chest X-ray was normal. Electroencephalography demonstrated diffuse, generalized slowing, and computed tomography revealed no acute intracranial pathology. Does this patient have neuroleptic malignant syndrome (NMS)? What are the risk factors for NMS? What are the most sensitive diagnostic criteria? What is known about the pathophysiology of this condition? What treatment strategies are available and what treatments should be initiated?
Scope and Nature of Neuroleptic Malignant Syndrome
NMS, first described nearly five decades ago, is an idiosyncratic, life-threatening complication of treatment with antipsychotic drugs that is characterized by fever, severe muscle rigidity, and autonomic and mental status changes (1 , 2) . Although estimates of the incidence of NMS once ran as high as 3% of patients treated with antipsychotics, more recent data suggest an incidence of 0.01%–0.02% (3) . This decrease in frequency likely reflects increased awareness of the disorder, more conservative prescribing patterns, and the shift to use of atypical antipsychotics. In addition, progression to more fulminant, lethal NMS episodes may occur less often because of widespread recognition and earlier diagnosis of this drug-induced reaction. Despite its declining frequency, however, NMS remains a significant source of morbidity and mortality among patients receiving antipsychotics. For example, data from the U.S. Agency for Healthcare Research and Quality indicate that about 2,000 cases of NMS are diagnosed annually in hospitals in the United States, incurring health care costs of $70 million, with a mortality rate of 10%, which underscores the continuing public health impact of NMS (http://hcup.ahrq.gov/HCUPnet.asp).
Diagnosis
Despite the availability of operational criteria (4 , 5) , NMS is often difficult to distinguish from more common extrapyramidal side effects of antipsychotics and from other disorders presenting with similar symptoms (6 – 8) . DSM-IV-TR research criteria require that both severe muscle rigidity and elevated temperature be present after recent administration of an antipsychotic as well as two associated signs, symptoms, or laboratory findings that are not better accounted for by a substance-induced, neurological, or general medical condition. Rating scales have been introduced for tracking the clinical course of NMS on the basis of factors such as severity of hyperthermia, rigidity, mental status alteration, and elevation in serum creatine kinase (9 , 10) .
Laboratory investigations are essential to exclude other disorders or complications. Several laboratory abnormalities are associated with NMS, although none are specific for the diagnosis (7 , 8) . For example, patients with NMS may have rhabdomyolysis, resulting in significant increases in serum creatine kinase, aldolase, transaminases, and lactic acid dehydrogenase concentrations, with the risk of subsequent myoglobinuric renal failure. Patients may also have metabolic acidosis, hypoxia, decreased serum iron concentrations, elevated serum catecholamines, and leukocytosis, with or without left shift. Results of CSF analysis are normal in more than 95% of cases (11) . Findings of neuroimaging studies are generally within normal limits, and electroencephalography may demonstrate generalized slowing consistent with metabolic encephalopathy (11) .
The temporal progression of signs and symptoms may provide important clues to diagnosis and severity of illness. Retrospective analyses suggest that alteration in mental status and other neurological signs precede systemic signs in more than 80% of cases of NMS (8 , 12) . Although the initial progression of symptoms is usually insidious over days, occasional cases of NMS may have a fulminant onset within hours after drug administration. About 16% of cases of NMS develop within 24 hours after initiation of antipsychotic treatment, 66% within the first week, and virtually all cases within 30 days (11) . It would be unusual for NMS to occur beyond 1 month after initiation of treatment unless the dose was increased or an additional antipsychotic administered. Once NMS is diagnosed and oral antipsychotic drugs are discontinued, NMS is self-limited in most cases. The mean recovery time after drug discontinuation is in the range of 7–10 days, with 63% of patients recovering within 1 week and nearly all within 30 days (11) . However, the duration of NMS episodes may be prolonged when long-acting depot antipsychotics are implicated. In addition, there have been several reports of patients in whom residual catatonia and parkinsonism persisted for weeks after the acute metabolic symptoms of NMS resolved (8 , 13) . Clinicians should bear in mind that although NMS is striking in its classic form, the condition is heterogeneous in onset, presentation, progression, and outcome.
Differential Diagnosis
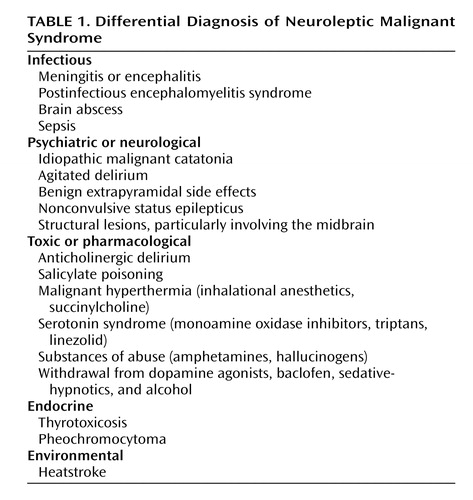
Differential diagnosis ( Table 1 ) is of prime importance because NMS is a diagnosis of exclusion. Central, systemic, and toxic causes of hyperthermia and rigidity must be excluded, as well as other causes of rhabdomyolysis and altered mental status. According to a compilation of cases reported to the Neuroleptic Malignant Syndrome Information Service, infections, agitated delirium, and benign extrapyramidal symptoms are among the processes most commonly confused with NMS (S.N. Caroff, unpublished data, 2007).
In the differential diagnosis, special attention should be given to the evaluation of CNS infections, especially viral encephalitis, which can be difficult to distinguish from NMS. Prodromal viral illnesses, headaches, meningeal signs, seizures, localizing neurological signs, CSF studies, and neuroimaging may suggest an infectious etiology. Caroff and Mann (7) noted that the risk of severe drug-induced extrapyramidal reactions, including NMS, may be heightened in patients infected by HIV and other viruses that affect midbrain structures. Anatomic lesions affecting midbrain and brainstem structures, as well as rare cases of nonconvulsive status epilepticus, can simulate NMS and are considered in the differential diagnosis.
Advanced stages of psychotic disorders associated with excited or stuporous catatonia (delirious mania and malignant catatonia) can present with hyperthermia and appear indistinguishable from NMS (14) . Indeed, NMS has been conceptualized as a drug-induced iatrogenic form of malignant catatonia (14 , 15) . Although some features—such as parkinsonian symptoms; extreme hyperthermia and stupor developing only after drug administration; absence of an underlying psychiatric disorder; and so on—may be suggestive of drug-induced malignant catatonia (i.e., NMS) rather than idiopathic malignant catatonia due to progression of psychotic illness, the two conditions may be indistinguishable in more than 20% of cases and may reflect the same underlying pathophysiology (14) . In either NMS or malignant catatonia due to psychosis, antipsychotics should be discontinued; most NMS episodes are self-limited once medication is stopped, and in idiopathic malignant catatonia, antipsychotics appear to be ineffective or even detrimental. ECT appears to be the treatment of choice in malignant catatonia, and it is often effective in NMS as well.
Among systemic disorders, heatstroke can present with hyperthermia, confusion, tachycardia, and tachypnea, and its differentiation from NMS may be difficult in a psychiatric patient receiving antipsychotic medication. However, in heatstroke patients, in addition to a history of exertion or exposure to high ambient temperatures, the skin is dry and muscle flaccidity is commonly observed.
Several classes of drugs may cause symptoms resembling those of NMS. Dopamine antagonists other than antipsychotic drugs (e.g., metoclopramide, amoxapine, and prochlorperazine) have reportedly caused NMS. Withdrawal of dopaminergic agents (e.g., amantadine and l -dopa) or of the GABA-ergic drug baclofen can precipitate an NMS-like reaction. Serotonergic drugs, including selective serotonin reuptake inhibitors, tricyclic antidepressants, monoamine oxidase inhibitors (including linezolid), and triptans used to treat migraine headaches, can cause serotonin syndrome, which most often presents as an agitated delirium but resembles NMS in severe cases. It is important to differentiate between serotonin syndrome and NMS not only because the treatment approaches for the two conditions may differ but also because the diagnosis will affect how one approaches resumption of antipsychotic medication in patients with persistent or recurrent psychosis.
Patients undergoing general anesthesia may develop the NMS-like signs of malignant hyperthermia. In contrast to NMS, these patients usually develop symptoms intraoperatively, have a primary pharmacogenetic skeletal muscle disorder (which consequently is not relieved by neuromuscular blocking agents), and may have a family history of malignant hyperthermia during surgery (8 , 16) .
Certain substances of abuse are associated with NMS-like presentations, among them cocaine and amphetamine (especially Ecstasy [3,4-methylenedioxymethamphetamine, or MDMA]). Hallucinogen intoxication (e.g., from phencyclidine) and withdrawal from alcohol and sedative-hypnotics also may cause fever, autonomic changes, and other symptoms that can be confused with NMS.
Risk Factors
Several studies of risk factors for NMS (17) suggest that age, sex, and time of year are not significantly correlated with risk of developing the condition. NMS is not specific to any neuropsychiatric diagnosis, although patients with catatonia may be at risk of progressing to NMS after receiving antipsychotics.
Several clinical, systemic, and metabolic factors have been correlated with the incidence of NMS, including agitation, dehydration, restraint, preexisting abnormalities of CNS dopamine activity or receptor function, and iron deficiency (18 , 19) . Nearly all case series of NMS patients have reported physical exhaustion and dehydration prior to the onset of NMS (17) . Elevated environmental temperature has been proposed as a contributing factor in some series, although NMS can occur independent of ambient conditions. A prior episode of NMS has been described in 15%–20% of cases (8 , 11) .
Pharmacological and treatment variables have been examined as risk factors for NMS. Nearly all dopamine antagonists have been associated with NMS, although high-potency conventional antipsychotics are associated with a greater risk compared with low-potency agents and atypical antipsychotics (3 , 7) . Parenteral routes, higher titration rates, and total dose of drug administration have been associated with an increased risk of NMS (17) ; however, a significant number of NMS cases occur at therapeutic doses of these agents. Although cases of NMS meeting DSM-IV-TR research criteria have been reported with clozapine, olanzapine, and risperidone, unequivocal cases implicating monotherapy with quetiapine, ziprasidone, or aripiprazole remain scarce (20) .
Although evidence from small cohort studies suggests that these clinical and pharmacological variables correlate with the risk of NMS, they are not practical in predicting risk in a given patient because they are relatively common and NMS is relatively uncommon. In other words, the association of these risk factors with NMS in a few patients may not outweigh the benefits of antipsychotics for the vast majority of psychotic patients.
Pathophysiology
Although the precise pathophysiological mechanisms of NMS are unproven, antipsychotic-induced dopamine blockade likely plays a pivotal triggering role in the condition ( Figure 1 ) (21) . This hypothesis is supported by several lines of evidence: withdrawal of dopaminergic drugs can precipitate an NMS-like syndrome; all drugs associated with NMS produce dopamine receptor blockade; the risk of NMS appears to be correlated with the dopamine-receptor-binding affinity of drugs; dopaminergic drugs have been used in treatment of NMS symptoms; and patients with central dopamine tract lesions have been noted to develop syndromes that share many clinical characteristics with NMS. The central role of dopaminergic hypofunction is further supported by the observation that the CSF concentration of the dopamine metabolite homovanillic acid is low in patients with acute NMS (22) . A number of preliminary studies have searched for polymorphisms within the dopamine 2 receptor gene in patients who have recovered from NMS, although results have not been consistent (8) .
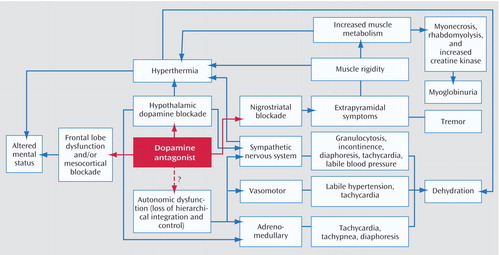
a Adapted from Gurrera (24).
Based on the autonomic dysfunction described over the past two decades in NMS and the observation that catecholamine levels are elevated in many cases, sympathoadrenal dysfunction has been suggested as having a contributing role in NMS (23 , 24) . Whatever the initiating mechanism, the pathophysiology of NMS is likely complex, involving a cascade of dysregulation in multiple neurochemical and neuroendocrine systems culminating in an end-stage hypermetabolic syndrome.
Treatment and Management
Supportive Therapy
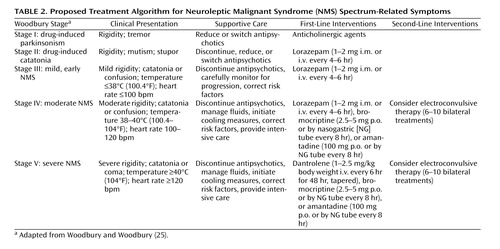
The offending agent must be withdrawn immediately, after which supportive medical therapy is the mainstay of management of NMS (25 , 26) . Table 2 presents a treatment algorithm for NMS, including clinical presentation by illness stage or severity. Volume resuscitation should be aggressive, especially given that most patients with NMS are dehydrated in the acute phase of the illness. Serial monitoring and correction of electrolyte abnormalities is critical. Recent reports suggest that alkalinized fluids or even bicarbonate loading may be of particular benefit in preventing renal failure (27) . In extreme hyperthermia, physical cooling measures are paramount, as the peak and duration of temperature elevation are predictive of morbidity and mortality (8) . Intensive medical care should include careful monitoring for complications, including cardiorespiratory failure, renal failure, aspiration pneumonia, and coagulopathies, and may involve support of cardiac, respiratory, and renal function.
Pharmacological Treatments
NMS is a self-limited iatrogenic disorder, and in many cases medical management and cessation of antipsychotic medication may suffice to reverse the symptoms. There is no general consensus on specific pharmacological treatments for uncomplicated NMS, and there is only limited evidence on whether specific remedies can facilitate recovery and improve outcome. It is difficult to compare specific treatments for NMS because the disorder is rare, heterogeneous, and unpredictable in onset and progression, which precludes randomized controlled studies. However, theoretical grounds and numerous clinical reports provide some support for several empirical, off-label treatment approaches (26) .
Benzodiazepines
Although a controlled evaluation of NMS risk factors suggests that benzodiazepines do not have a preventive effect (17) , several clinical reports suggest that benzodiazepines, administered orally or parenterally, may ameliorate symptoms and hasten recovery in NMS, particularly in milder cases. This observation is not surprising given that NMS has been considered an extreme form of catatonia (25 , 28) . However, there have been reports of cases of acute NMS in which benzodiazepines had no clinical effect or produced only transient clinical improvement. Nonetheless, given the relative risks and benefits, a trial of lorazepam, starting with 1–2 mg parenterally, is a reasonable first-line intervention in patients with acute NMS, particularly in those with milder and primarily catatonic symptoms.
Dopaminergic Agents
Several dopaminergic drugs, including bromocriptine and amantadine, may reverse parkinsonism in NMS and have been reported in case reports and meta-analyses (8 , 29 , 30) to reduce time to recovery and halve mortality rates when used alone or in combination with other treatments. Amantadine is generally initiated at 200–400 mg/day in divided doses administered orally or through a nasogastric tube. The starting dose of bromocriptine is 2.5 mg orally two or three times a day, increased to a total daily dose of 45 mg if necessary. Bromocriptine can worsen psychosis and hypotension. It also may precipitate vomiting and thus should be used carefully in patients at risk of aspiration. Premature discontinuation of bromocriptine has resulted in rebound symptoms in some cases.
Dantrolene
Because of its efficacy in anesthetic-induced malignant hyperthermia, the muscle relaxant dantrolene has been used in the treatment of NMS. Dantrolene may be useful only in cases of NMS with extreme temperature elevations, rigidity, and true hypermetabolism (8) . Generally, rapid reversal of the hyperthermia and rigidity is observed in patients treated with dantrolene, but symptoms may return if treatment is discontinued prematurely. Dantrolene can be combined with benzodiazepines or dopamine agonists, but it should not be coadministered with calcium channel blockers, as cardiovascular collapse can occur. Typical dosing of intravenous dantrolene in the treatment of NMS is 1–2.5 mg/kg body weight administered initially, followed by 1 mg/kg every 6 hours if rapid resolution of the fever and rigidity is observed, with tapering or switching to oral dantrolene after the first few days. Side effects may include impairment of respiratory or hepatic function. In some meta-analyses (8 , 29 , 30) , improvement has been reported in approximately 80% of NMS patients treated with dantrolene monotherapy. In addition, time to recovery may be shortened, and mortality is decreased by nearly half compared with supportive care, whether dantrolene is used alone or in combination with other agents. However, other anecdotal reports and a recent meta-analysis of published cases did not support the efficacy of dantrolene in NMS (31) .
ECT
As the above suggests, pharmacotherapy has not been consistently effective in all case reports of NMS. Moreover, drug effects are usually observed early and are unlikely to occur after the first few days of treatment. In contrast, ECT may be effective if symptoms are refractory to supportive care and pharmacotherapy even late in the course of NMS, or if idiopathic malignant catatonia due to an underlying psychotic disorder cannot be excluded, or if the patient has persistent residual catatonia and parkinsonism after resolution of the acute metabolic symptoms of NMS.
A review (32) found that ECT was consistently effective even after failed pharmacotherapy and that clinical response often occurred over the course of the first several treatments. Treatment response to ECT was not predicted by age, sex, psychiatric diagnosis, or any particular features of NMS. A typical ECT regimen for acute NMS would include six to 10 treatments with bilateral electrode placement. ECT is a relatively safe treatment in NMS, although use of succinylcholine during anesthesia should be carefully considered in patients with severe rhabdomyolysis to avoid the risk of hyperkalemia and cardiovascular complications.
Antipsychotic Use Following NMS
Restarting antipsychotic treatment after resolution of an NMS episode has been associated with an estimated likelihood of developing NMS again as high as 30% (11 , 33) . Nevertheless, most patients who require antipsychotic treatment can be safely treated, provided precautions are taken (7) . For example, reports of previous episodes should be checked for accuracy; indications for antipsychotics should be clearly documented; alternative medications should be considered; risk factors should be reduced; at least 2 weeks should be allowed to elapse after recovery from NMS before rechallenge; low doses of low-potency conventional antipsychotics or atypical antipsychotics should be titrated gradually after a test dose; and patients should be carefully monitored for early signs of NMS. In addition, prudence dictates that documented informed consent should be obtained from patients and family members regarding the benefits of restarting antipsychotic therapy versus the risk of recurrence of NMS. There are no data on rechallenging patients who recover from idiopathic malignant catatonia with antipsychotics, but following the same precautions as for NMS would be sensible.
Summary and Recommendations
The incidence of NMS is estimated at 0.01%–0.02% of patients treated. Although the widespread adoption of atypical antipsychotics has markedly reduced the risk of neurological disorders, NMS remains a risk for susceptible patients receiving these drugs. The atypical agents are associated with less risk of NMS than the conventional antipsychotics. Nevertheless, clinicians must be aware of the clinical features of NMS and vigilant in detecting early signs. Primary management of NMS lies in prevention through conservative use of antipsychotics, reduction of risk factors, early diagnosis, prompt discontinuation of offending medications, and medical management. In the absence of randomized controlled trials, it may be unwarranted to recommend one single intervention over another or over supportive management. Specific treatment of NMS should be individualized and based empirically on the character, duration, and severity or stage of clinical signs and symptoms (25 , 26) . For mild cases, supportive care and careful clinical monitoring may be sufficient (7 , 8) , whereas in severe cases, more aggressive measures should be taken, including empirical trials of specific pharmacological agents or ECT ( Table 2 ).
The patient in the vignette is suffering from severe (stage V) NMS. All antipsychotic medications should be stopped immediately, and cooling measures and aggressive medical management, including intravenous fluids, should be initiated in an intensive care setting. Lorazepam or dopaminergic agents could be tried empirically. However, given the risks of extreme hyperthermia and rigidity associated with significant rhabdomyolysis in this case, intravenous dantrolene could be administered for 48 hours, followed by tapering if the fever and rigidity resolve. If Ms. A’s symptoms do not improve after several days, ECT should be considered.
Approximately 2 weeks after resolution of NMS, treatment with a low-potency atypical antipsychotic should be initiated at a low dose and slowly titrated in a monitored setting with careful assessment for signs of recurrent NMS.
1. Delay J, Pichot P, Lempérière T, Elissade B, Peigne F: Un neuroleptique majeur non-phénothiazine et nonréserpinique, l’halopéridol, dans le traitement des psychoses. Annales Médico-Psychologique 1960; 118:145–152Google Scholar
2. Caroff SN: The neuroleptic malignant syndrome. J Clin Psychiatry 1980; 41:79–83Google Scholar
3. Stubner S, Rustenbeck E, Grohmann R, Wagner G, Engel R, Neundorfer G, Moller HJ, Hippius H, Ruther E: Severe and uncommon involuntary movement disorders due to psychotropic drugs. Pharmacopsychiatry 2004; 37(suppl 1):S54–S64Google Scholar
4. Pope HG Jr, Keck PE Jr, McElroy SL: Frequency and presentation of neuroleptic malignant syndrome in a large psychiatric hospital. Am J Psychiatry 1986; 143:1227–1233Google Scholar
5. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, American Psychiatric Association, 1994Google Scholar
6. Strawn JR: Aripiprazole and the neuroleptic malignant syndrome. Schizophr Res 2006; 85:298–299Google Scholar
7. Caroff SN, Mann SC: Neuroleptic malignant syndrome. Med Clin North Am 1993; 77:185–202Google Scholar
8. Caroff SN: Neuroleptic malignant syndrome, in Neuroleptic Malignant Syndrome and Related Conditions, 2nd ed. Edited by Mann SC, Caroff SN, Keck PE Jr, Lazarus A. Washington, DC, American Psychiatric Publishing, 2003, pp 1–44Google Scholar
9. Yacoub A, Kohen I, Caraballo A, Francis A: Rating Scale for Neuroleptic Malignant Syndrome. Biol Psychiatry 2004; 55:89SGoogle Scholar
10. Sachdev PS: A rating scale for neuroleptic malignant syndrome. Psychiatry Res 2005; 135:249–256Google Scholar
11. Caroff SN, Mann SC: Neuroleptic malignant syndrome. Psychopharmacol Bull 1988; 24:25–29Google Scholar
12. Velamoor VR, Norman RM, Caroff SN, Mann SC, Sullivan KA, Antelo RE: Progression of symptoms in neuroleptic malignant syndrome. J Nerv Ment Dis 1994; 182:168–173Google Scholar
13. Caroff SN, Mann SC, Keck PE Jr, Francis A: Residual catatonic state following neuroleptic malignant syndrome. J Clin Psychopharmacol 2000; 20:257–259Google Scholar
14. Mann SC, Caroff SN, Bleier HR, Welz WK, Kling MA, Hayashida M: Lethal catatonia. Am J Psychiatry 1986; 143:1374–1381Google Scholar
15. Fink M: Neuroleptic malignant syndrome and catatonia: one entity or two? Biol Psychiatry 1996; 39:1–4Google Scholar
16. Caroff SN, Rosenberg H, Mann SC, Campbell EC, Gliatto MF, Sullivan KA: Neuroleptic malignant syndrome in the perioperative setting. Am J Anesthesiol 2001; 28:387–393Google Scholar
17. Keck PE Jr, Pope HG Jr, Cohen BM, McElroy SL, Nierenberg AA: Risk factors for neuroleptic malignant syndrome. Arch Gen Psychiatry 1989; 46:914–918Google Scholar
18. Rosebush PI, Mazurek MF: Serum iron and neuroleptic malignant syndrome. Lancet 1991; 338:149–151Google Scholar
19. Lee JW: Serum iron in catatonia and neuroleptic malignant syndrome. Biol Psychiatry 1998; 44:499–507Google Scholar
20. Ananth J, Parameswaran S, Gunatilake S, Burgoyne K, Sidhom T: Neuroleptic malignant syndrome and atypical antipsychotic drugs. J Clin Psychiatry 2004; 65:464–470Google Scholar
21. Mann SC, Caroff SN, Fricchione G, Campbell EC: Central dopamine hypoactivity and the pathogenesis of the neuroleptic malignant syndrome. Psychiatr Ann 2000; 30:363–374Google Scholar
22. Nisijima K, Ishiguro T: Cerebrospinal fluid levels of monoamine metabolites and gamma-aminobutyric acid in neuroleptic malignant syndrome. J Psychiatr Res 1995; 27:233–244Google Scholar
23. Feibel JH, Schiffer RB: Sympathoadrenomedullary hyperactivity in the neuroleptic malignant syndrome: a case report. Am J Psychiatry 1981; 138:1115–1116Google Scholar
24. Gurrera RJ: Sympathoadrenal hyperactivity and the etiology of neuroleptic malignant syndrome. Am J Psychiatry 1999; 156:169–180Google Scholar
25. Woodbury MM, Woodbury MA: Neuroleptic-induced catatonia as a stage in the progression toward neuroleptic malignant syndrome. J Am Acad Child Adolesc Psychiatry 1992; 31:1161–1164Google Scholar
26. Davis JM, Caroff SN, Mann SC: Treatment of neuroleptic malignant syndrome. Psychiatr Ann 2000; 30:325–331Google Scholar
27. Strawn JR, Keck PE Jr: Early bicarbonate loading and dantrolene for ziprasidone/haloperidol-induced neuroleptic malignant syndrome (letter). J Clin Psychiatry 2006; 67:677Google Scholar
28. Francis A, Koch M, Chandragiri S, Rizvi S, Petrides G: Is lorazepam a treatment for neuroleptic malignant syndrome? CNS Spectr 2000; 5:54–57Google Scholar
29. Sakkas P, Davis JM, Janicak PG, Wang ZY: Drug treatment of the neuroleptic malignant syndrome. Psychopharmacol Bull 1991; 27:381–384Google Scholar
30. Rosenberg MR, Green M: Neuroleptic malignant syndrome: review of response to therapy. Arch Intern Med 1989; 149:1927–1931Google Scholar
31. Reulbach U, Dutsch C, Biermann T, Sperling W, Thuerauf N, Kornhuber J, Bleich S: Managing an effective treatment for neuroleptic malignant syndrome. Crit Care 2007; 11:R4 (epub Jan 12, 2007)Google Scholar
32. Troller JN, Sachdev PS: Electroconvulsive treatment of neuroleptic malignant syndrome: a review and report of cases. Aust NZ J Psychiatry 1999; 33:650–659Google Scholar
33. Pope HG Jr, Aizley HG, Keck PE Jr, McElroy SL: Neuroleptic malignant syndrome: long-term follow-up of 20 cases. J Clin Psychiatry 1991; 52:208–212Google Scholar