A Comparison of Medication Side Effect Reports by Panic Disorder Patients With and Without Concomitant Cognitive Behavior Therapy
Abstract
Objective: The authors assessed whether adding cognitive behavior therapy (CBT) to imipramine for patients with panic disorder decreased the severity of side effects and dropouts from side effects. Method: Data were analyzed for 172 panic disorder patients who were randomly assigned to receive imipramine alone, imipramine plus CBT, or placebo. Mixed-effects models were used to assess longitudinal differences among the treatment groups with respect to side effect burden and dropout rates during the acute, maintenance, and follow-up phases of treatment. Results: Patients treated with imipramine plus CBT experienced less severe fatigue/weakness, dry mouth, and sweating and had a lower rate of dropout due to side effects compared with those treated with imipramine only. Conclusions: The addition of CBT to medication treatment with imipramine was associated with less severe side effects and fewer dropouts due to perceived side effects than treatment with imipramine alone.
Panic disorder patients are fearful of somatic sensations, and intolerance of side effects is a consequence of this fear. Rejection of medication due to side effects is one of the major obstacles to successful pharmacotherapy in patients with panic disorder (1) . Dropout due to side effects can lead to bias even in randomized trials if dropout rates differ across groups (2) . Therefore, it is important to identify methods that will reduce the incidence and severity of side effects in clinical trials, both to enhance the trials’ ability to show superiority of active drugs compared with placebo and to improve the clinical outcomes of patients with conditions such as panic disorder. We investigated whether patients with panic disorder who received cognitive behavior therapy (CBT) plus imipramine reported less severe medication-related side effects and were less likely to drop out because of side effects compared with patients who received imipramine alone.
Method
A total of 312 patients meeting DSM-III-R criteria for panic disorder were randomly assigned to receive imipramine alone, imipramine plus CBT, placebo alone, CBT alone, or CBT plus placebo (see reference 3 for more detail). In this analysis, we used data for 172 subjects: those from the imipramine only group (N=83), the imipramine plus CBT group (N=65), and the placebo only group (N=24).
The study was divided into three treatment phases: a 12-week acute phase in which patients were seen 10 times for treatment sessions; a 6-month maintenance phase in which participants who responded to acute therapy were maintained on double-blind treatment and seen monthly; and a 6-month follow-up phase in which treatments were discontinued for participants who responded during the maintenance phase and participants were evaluated every month. Patients who were assigned to any of the medication conditions received imipramine or matching placebo pills in a fixed flexible-dose design beginning at 25 mg daily and going as high as 300 mg daily, with an attempt to get all patients to at least 200 mg for at least the last 4 weeks of the acute phase.
At each medication visit, all patients were asked about the following side effects: insomnia, sleep disturbance, drowsiness, nervousness, fatigue, irritability, memory problems, impaired mentation, dizziness, headache, blurred vision, tinnitus, dry mouth, tremors, palpitations, abdominal discomfort, constipation, urination problems, menstrual irregularity, libido decrease, sexual dysfunction, sweating, appetite decrease, appetite increase, and weight gain. CBT did not focus on side effects but did address somatic panic symptoms.
We used mixed-effects models for ordinal outcomes to model side effect severity on an ordinal scale (0=none, 1=mild, 2=moderate, and 3=severe) as a function of session, CBT plus medication (yes or no), and placebo (yes or no). This parameterization allowed us to make the critical comparisons directly 1) between medication and medication plus CBT and 2) between medication and placebo. Mixed-effects ordinal regressions were performed using MIXOR (4; http://www.uic.edu/~hedeker/mix.htm). We used mixed-effects models because they allow for missing sessions and serial correlation due to repeated observations on the same individual (5) . With Bonferroni-type adjustments, by contrast, it would be easier to miss an important side effect, providing a less conservative analysis (6) .
Results
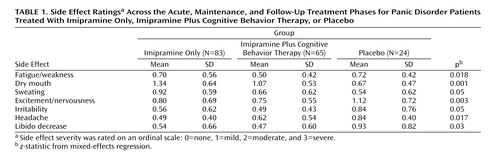
Table 1 presents the side effect ratings that had statistically significant differences between the three groups across the three treatment phases. Patients treated with imipramine plus CBT reported less fatigue/weakness, dry mouth, and sweating during the acute, maintenance, and follow-up treatment phases compared with those treated with imipramine alone. The placebo group reported greater levels of excitement/nervousness, irritability, headache, and libido decrease compared with the other two groups. These symptoms, however, were most likely due to panic disorder and associated anxiety rather than to medication.
Of the 172 patients in our sample, 89 dropped out during the acute or maintenance treatment phases, and of these, 16 patients (18%) dropped out because of side effects. In the imipramine only group, 11 of the original 83 patients (13%) dropped out because of side effects, all of them during the acute treatment phase. In the imipramine plus CBT group, two of the original 65 patients (3%) dropped out because of side effects during the acute phase and another five (8%) dropped out during the maintenance phase. There were no dropouts in these two groups during the follow-up phase, and no one in the placebo group dropped out because of side effects at any time. Across the three phases, the difference in dropout rates between the imipramine only group and the imipramine plus CBT group was significant (F=3.29, df=3, 167, p=0.02).
Discussion
We found that panic disorder patients treated with imipramine plus CBT reported significantly less severe side effects than patients treated with imipramine alone, and fewer dropped out because of side effects. Given our moderate sample size and the similar medication doses taken by those who completed treatment and those who dropped out, the differences between groups was striking. Nevertheless, our findings are limited by the study’s sample size and need to be confirmed with larger samples.
Side effects reported by patients with panic disorder cannot easily be distinguished from somatic symptoms associated with the underlying disorder (7) and are not necessarily true medication-related effects. In fact, we found that treatment with imipramine only or with imipramine plus CBT improved certain somatic symptoms compared with placebo. The CBT targeted fear of somatic sensations due to panic, and the resulting benefits may have not only reduced the perceived severity of medication side effects but also decreased the chances that a limited-symptom panic episode would be misperceived as a medication side effect.
Cognitive behavior panic control therapy (7) reduces the fear of sensations that is characteristic of panic disorder patients (8 , 9) . CBT also reduces vulnerability to sodium lactate-induced panic attacks (10) and may operate similarly to reduce sensitivity to medication-related somatic symptoms. Although we did not find better efficacy for imipramine plus CBT than for imipramine alone (3) , the differences we observed in perceived medication side effects and dropout rates, if confirmed, raise the possibility that combination treatment is in fact superior.
1. Cowley DS, Ha EH, Roy-Byrne PP: Determinants of pharmacologic treatment failure in panic disorder. J Clin Psychiatry 1997; 58:555–561Google Scholar
2. Marcus SM, Gorman JM, Tu X, Gibbons RD, Barlow DH, Woods SW, Shear MK: Rater bias in a blinded randomized placebo-controlled psychiatry trial. Stat Med 2006; 25:2762–2770Google Scholar
3. Barlow DH, Gorman JM, Shear MK, Woods SW: Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: a randomized controlled trial. JAMA 2000; 283:2529–2536Google Scholar
4. Hedeker D, Gibbons RD: MIXOR: a computer program for mixed-effects ordinal regression analysis. Comput Methods Programs Biomed 1996; 29:157–176Google Scholar
5. Gibbons RD: Mixed-effects models for mental health services research. Health Serv Outcomes Res Methodol 2000; 1:91–129Google Scholar
6. Marcus S: Multiple comparison in clinical trials: when to adjust, in 1993 Proceedings of the Biopharmaceutical Section of the American Statistical Association. Alexandria, Va, American Statistical Association, 1993, pp 398–400Google Scholar
7. Barlow DH: Cognitive-behavioral therapy for panic disorder: current status. J Clin Psychiatry 1997; 58(suppl 2):32–36Google Scholar
8. Bouton M, Mineka S, Barlow D: A modern learning theory perspective on the etiology of panic disorder. Psychol Rev 2001; 108:4–32Google Scholar
9. Shear MK, Houck P, Greeno C, Masters S: Emotion-focused psychotherapy for patients with panic disorder. Am J Psychiatry 2001; 158:1993–1998Google Scholar
10. Shear MK, Fyer AJ, Ball G, Josephson S, Fitzpatrick M, Gitlin B, Frances AJ, Gorman J, Liebowitz M, Klein DF: Vulnerability to sodium lactate in panic disorder patients given cognitive-behavioral therapy. Am J Psychiatry 1991; 148:795–797Google Scholar